Abstract
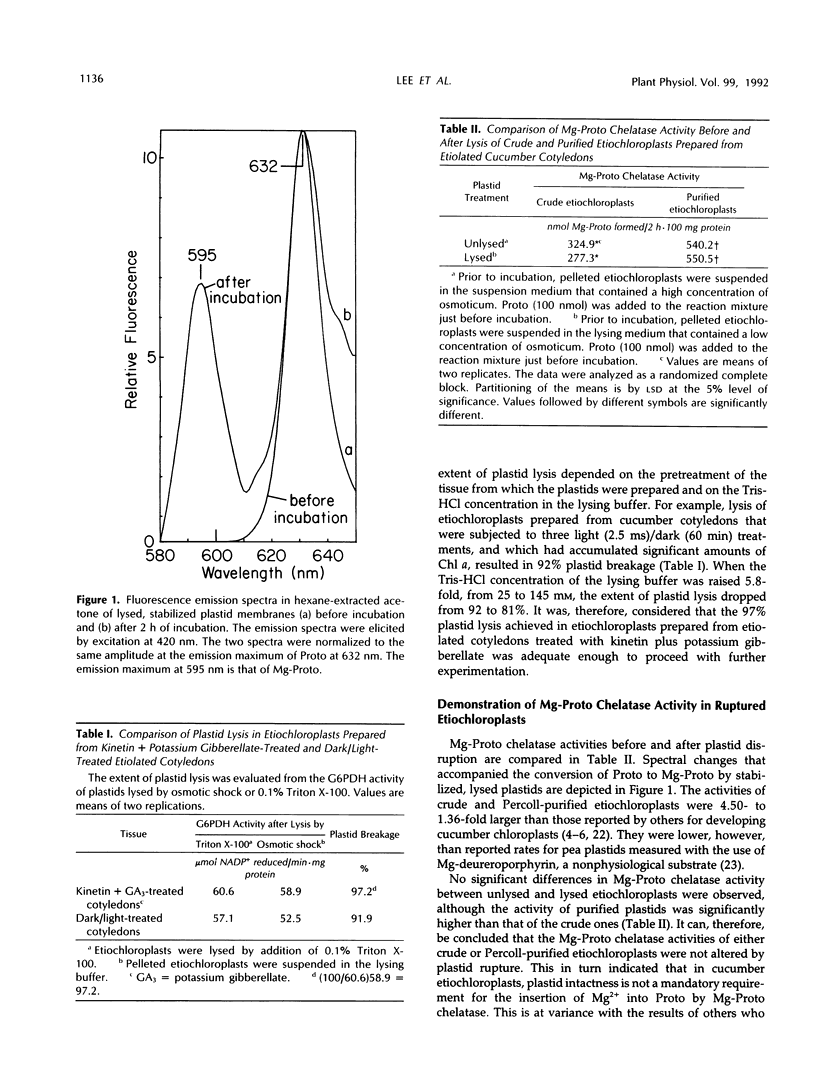
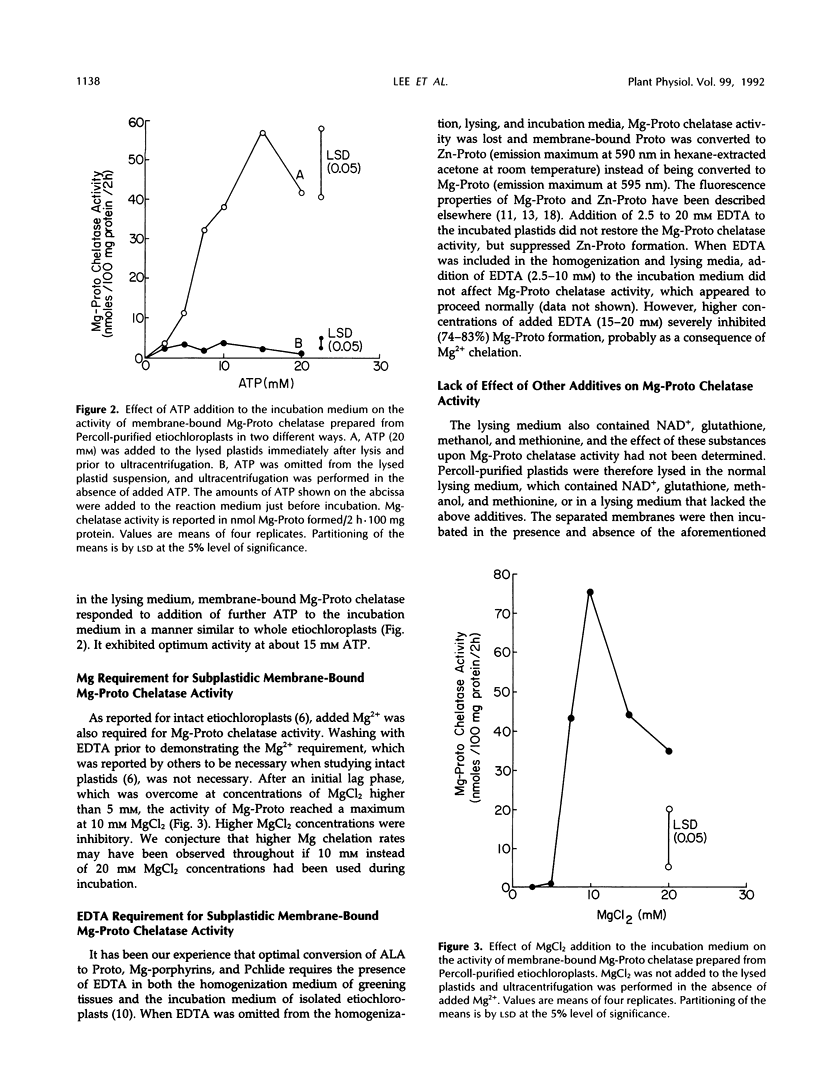
The preparation from Percoll-purified cucumber (Cucumis sativus)etiochloroplasts of a subplastidic membrane fraction that is capable of high rates of Mg insertion into protoporphyrin IX is described. The plastid stroma was inactive when used either alone or in combination with the membrane fraction. Successful preparation of the subplastidic membrane fraction required that Mg-protoporphyrin chelatase was first stabilized by its substrate. This was achieved by lysing Percoll-purified plastids in a fortified hypotonic medium containing protoporphyrin IX prior to ultracentrifugation and separation of the stroma from the plastid membranes. Protoporphyrin IX became membrane bound. Other additives needed for enzyme activity fell into two groups: (a) those needed for enzyme stabilization during membrane preparation and (b) those involved in the primary mechanism of Mg insertion into protoporphyrin IX. Ethylenediaminetetraacetate belonged to the first group, magnesium belonged to the second group, and ATP belonged to both groups.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Daniell H., Rebeiz C. A. Chloroplast culture. IX. Chlorophyll(ide) a biosynthesis in vitro at rates higher than in vivo. Biochem Biophys Res Commun. 1982 May 31;106(2):466–470. doi: 10.1016/0006-291x(82)91133-0. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P. Maintaining protein stability. Methods Enzymol. 1990;182:83–89. doi: 10.1016/0076-6879(90)82010-y. [DOI] [PubMed] [Google Scholar]
- Fuesler T. P., Wong Y. S., Castelfranco P. A. Localization of Mg-Chelatase and Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase Activities within Isolated, Developing Cucumber Chloroplasts. Plant Physiol. 1984 Jul;75(3):662–664. doi: 10.1104/pp.75.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Wright L. A., Castelfranco P. A. Properties of Magnesium Chelatase in Greening Etioplasts: METAL ION SPECIFICITY AND EFFECT OF SUBSTRATE CONCENTRATIONS. Plant Physiol. 1981 Feb;67(2):246–249. doi: 10.1104/pp.67.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufsler T. P., Castelfranco P. A., Wong Y. S. Formation of Mg-Containing Chlorophyll Precursors from Protoporphyrin IX, delta-Aminolevulinic Acid, and Glutamate in Isolated, Photosynthetically Competent, Developing Chloroplasts. Plant Physiol. 1984 Apr;74(4):928–933. doi: 10.1104/pp.74.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Ball M. D., Rebeiz C. A. Intraplastidic Localization of the Enzymes That Convert delta-Aminolevulinic Acid to Protoporphyrin IX in Etiolated Cucumber Cotyledons. Plant Physiol. 1991 Jul;96(3):910–915. doi: 10.1104/pp.96.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C. C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975 Dec;171(2):549–567. doi: 10.1016/0003-9861(75)90065-x. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of Mg-protoprophyrin IX monoester and longer wavelength metalloporphyrins by greening cotyledons. Arch Biochem Biophys. 1975 Feb;166(2):446–465. doi: 10.1016/0003-9861(75)90408-7. [DOI] [PubMed] [Google Scholar]
- Smith B. B., Rebeiz C. A. Chloroplast Biogenesis: XXIV. Intrachloroplastic Localization of the Biosynthesis and Accumulation of Protoporphyrin IX, Magnesium-Protoporphyrin Monoester, and Longer Wavelength Metalloporphyrins during Greening. Plant Physiol. 1979 Feb;63(2):227–231. doi: 10.1104/pp.63.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. B., Rebeiz C. A. Chloroplast biogenesis: detection of Mg-protoporphyrin chelatase in vitro. Arch Biochem Biophys. 1977 Apr 15;180(1):178–185. doi: 10.1016/0003-9861(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tripathy B. C., Rebeiz C. A. Chloroplast biogenesis. Demonstration of the monovinyl and divinyl monocarboxylic routes of chlorophyll biosynthesis in higher plants. J Biol Chem. 1986 Oct 15;261(29):13556–13564. [PubMed] [Google Scholar]
- Walker C. J., Weinstein J. D. Further characterization of the magnesium chelatase in isolated developing cucumber chloroplasts : substrate specificity, regulation, intactness, and ATP requirements. Plant Physiol. 1991 Apr;95(4):1189–1196. doi: 10.1104/pp.95.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. J., Weinstein J. D. In vitro assay of the chlorophyll biosynthetic enzyme Mg-chelatase: resolution of the activity into soluble and membrane-bound fractions. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5789–5793. doi: 10.1073/pnas.88.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]


