Abstract
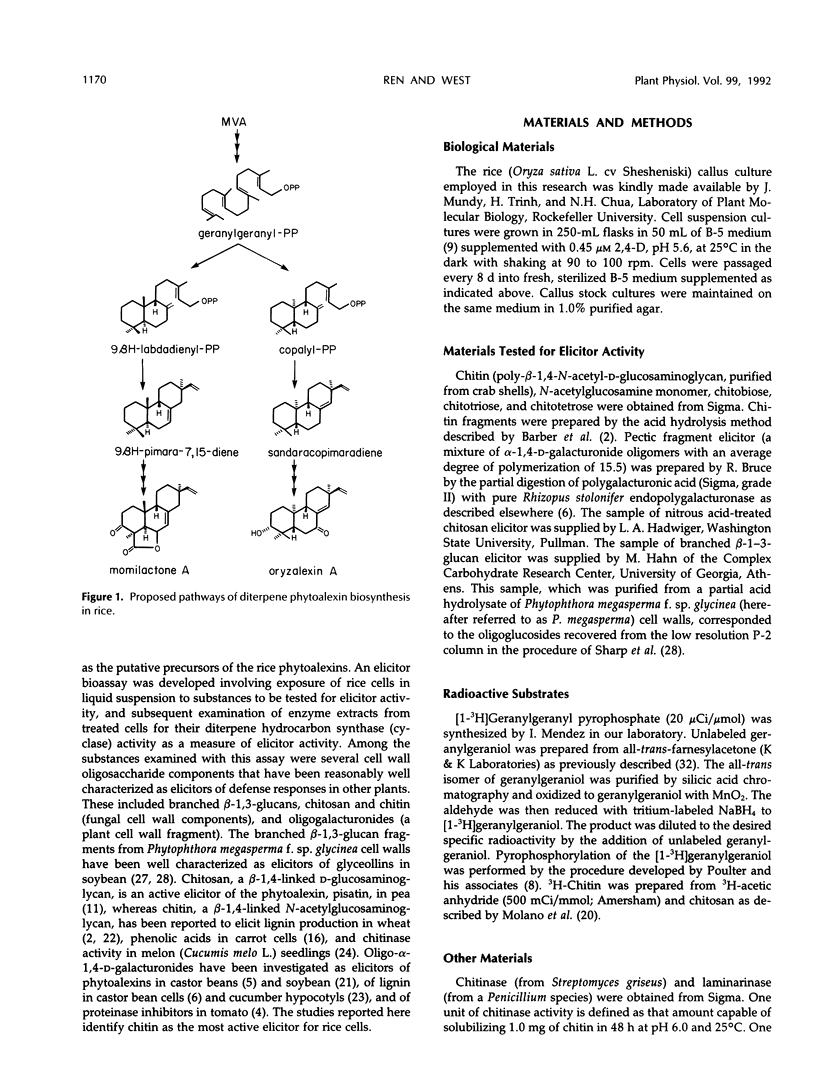
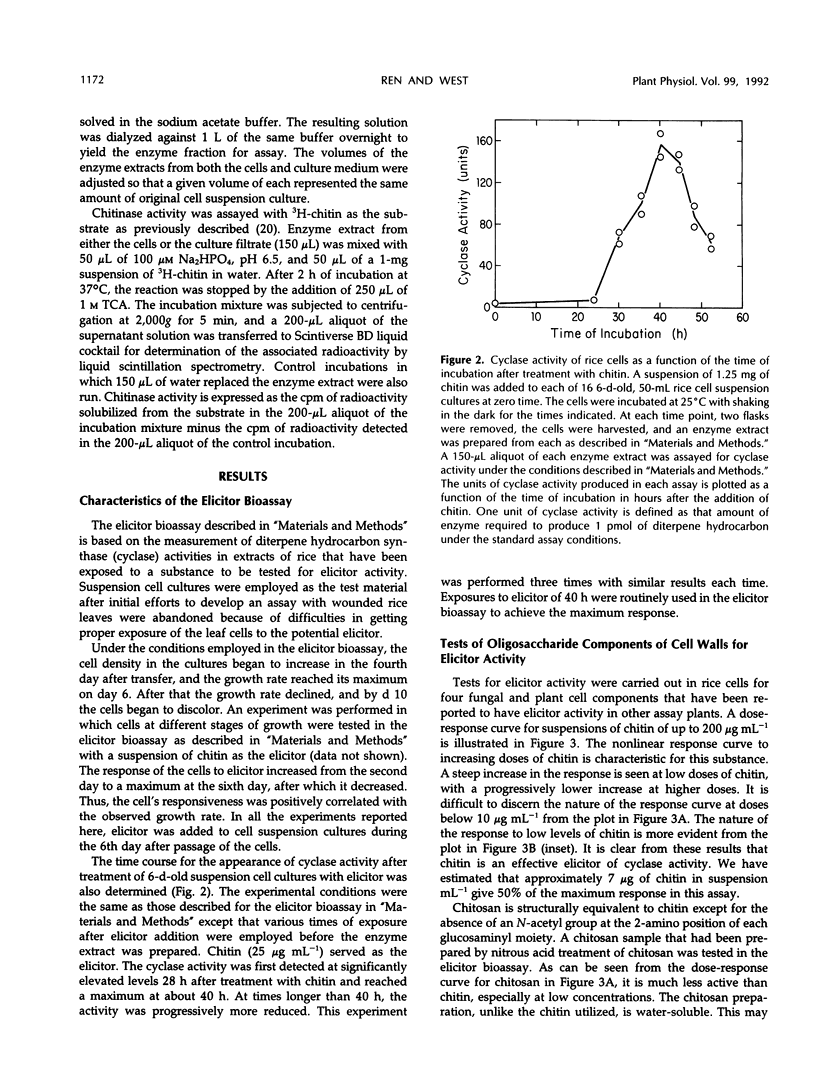
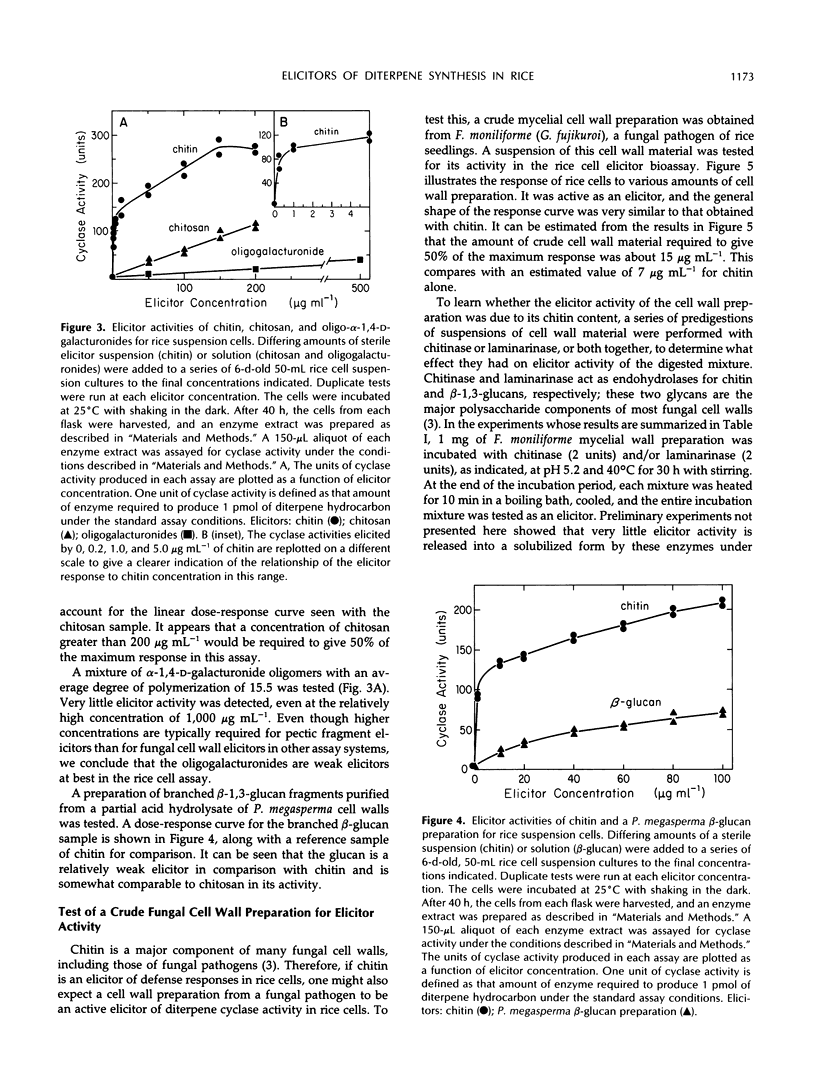
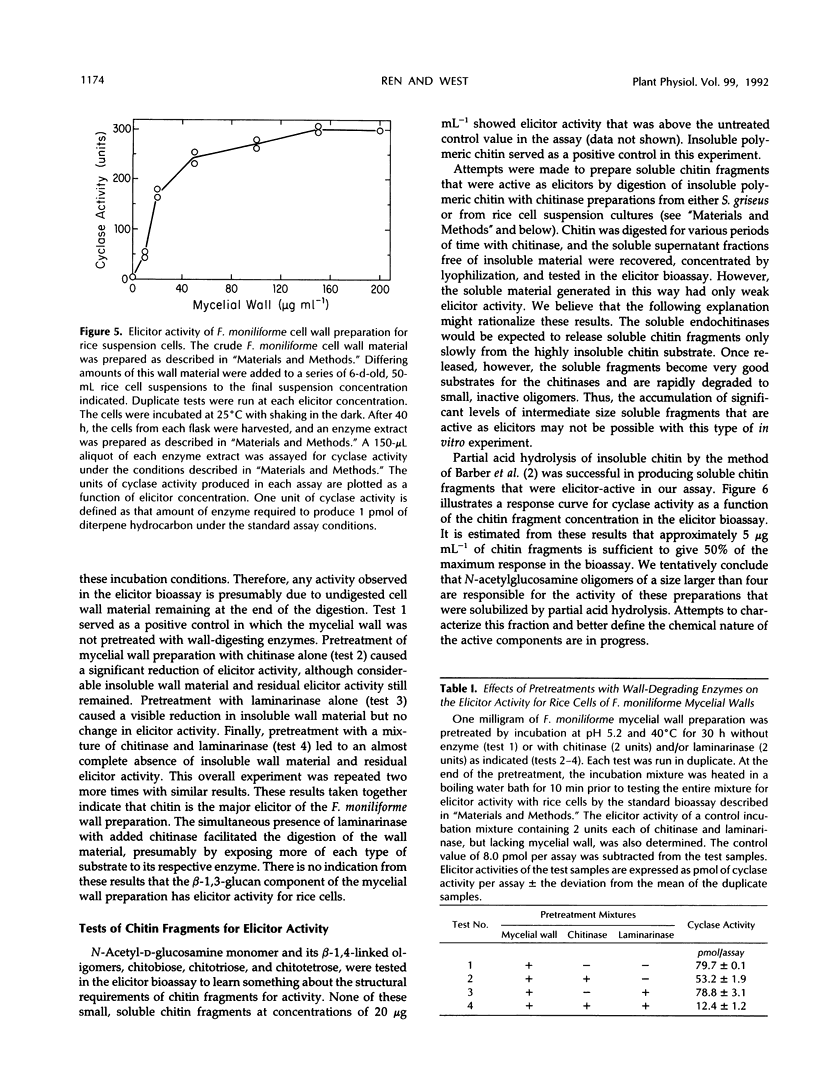
Cell-free extracts of UV-irradiated rice (Oryza sativa L.) leaves have a much greater capacity for the synthesis from geranylgeranyl pyrophosphate of diterpene hydrocarbons, including the putative precursors of rice phytoalexins, than extracts of unstressed leaves (KA Wickham, CA West [1992] Arch Biochem Biophys 293: 320-332). An elicitor bioassay was developed on the basis of these observations in which 6-day-old rice cell suspension cultures were incubated for 40 hours with the substance to be tested, and an enzyme extract of the treated cells was assayed for its diterpene hydrocarbon synthesis activity as a measure of the response to elicitor. Four types of cell wall polysaccharides and oligosaccharide fragments that have elicitor activity for other plants were tested. Of these, polymeric chitin was the most active; a suspension concentration of approximately 7 micrograms per milliliter gave 50% of the maximum response in the bioassay. Chitosan and a branched β-1,3-glucan fraction from Phytophthora megasperma f. sp. glycinea cell walls were only weakly active, and a mixture of oligogalacturonides was only slightly active. A crude mycelial cell wall preparation from the rice pathogen, Fusarium moniliforme, gave a response comparable to that of chitin, and this activity was sensitive to predigestion of the cell wall material with chitinase before the elicitor assay. N-Acetylglucosamine, chitobiose, chitotriose, and chitotetrose were inactive as elicitors, whereas a mixture of chitin fragments solubilized from insoluble chitin by partial acid hydrolysis was highly active. Constitutive chitinase activity was detected in the culture filtrate and enzyme extract of cells from a 6-day-old rice cell culture; the amount of chitinase activity increased markedly in both the culture filtrate and cell extracts after treatment of the culture with chitin. We propose on the basis of these results that soluble chitin fragments released from fungal cell walls through the action of constitutive rice chitinases serve as biotic elicitors of defense-related responses in rice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of Casbene Synthetase Activity in Castor Bean : THE ROLE OF PECTIC FRAGMENTS OF THE PLANT CELL WALL IN ELICITATION BY A FUNGAL ENDOPOLYGALACTURONASE. Plant Physiol. 1982 May;69(5):1181–1188. doi: 10.1104/pp.69.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989 Nov;91(3):889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davisson V. J., Woodside A. B., Poulter C. D. Synthesis of allylic and homoallylic isoprenoid pyrophosphates. Methods Enzymol. 1985;110:130–144. doi: 10.1016/s0076-6879(85)10068-6. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M. C., Forsyth W., Duncan H. J. A survey of the pectic content of nonlignified monocot cell walls. Plant Physiol. 1988 Oct;88(2):309–314. doi: 10.1104/pp.88.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Mauch-Mani B., Boller T. Antifungal Hydrolases in Pea Tissue : II. Inhibition of Fungal Growth by Combinations of Chitinase and beta-1,3-Glucanase. Plant Physiol. 1988 Nov;88(3):936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano J., Durán A., Cabib E. A rapid and sensitive assay for chitinase using tritiated chitin. Anal Biochem. 1977 Dec;83(2):648–656. doi: 10.1016/0003-2697(77)90069-0. [DOI] [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Gadelle A., Toppan A. Chitin oligosaccharides as elicitors of chitinase activity in melon plants. Biochem Biophys Res Commun. 1987 Mar 30;143(3):885–892. doi: 10.1016/0006-291x(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Sharp J. K., McNeil M., Albersheim P. The primary structures of one elicitor-active and seven elicitor-inactive hexa(beta-D-glucopyranosyl)-D-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J Biol Chem. 1984 Sep 25;259(18):11321–11336. [PubMed] [Google Scholar]
- Sharp J. K., Valent B., Albersheim P. Purification and partial characterization of a beta-glucan fragment that elicits phytoalexin accumulation in soybean. J Biol Chem. 1984 Sep 25;259(18):11312–11320. [PubMed] [Google Scholar]
- Skujins J. J., Potgieter H. J., Alexander M. Dissolution of fungal cell walls by a streptomycete chitinase and beta-(1-3) glucanase. Arch Biochem Biophys. 1965 Aug;111(2):358–364. doi: 10.1016/0003-9861(65)90197-9. [DOI] [PubMed] [Google Scholar]
- Upper C. D., West C. A. Biosynthesis of gibberellins. II. Enzymic cyclization of geranylgeranyl pyrophosphate to kaurene. J Biol Chem. 1967 Jul 25;242(14):3285–3292. [PubMed] [Google Scholar]
- Wickham K. A., West C. A. Biosynthesis of rice phytoalexins: identification of putative diterpene hydrocarbon precursors. Arch Biochem Biophys. 1992 Mar;293(2):320–332. doi: 10.1016/0003-9861(92)90402-i. [DOI] [PubMed] [Google Scholar]


