Abstract
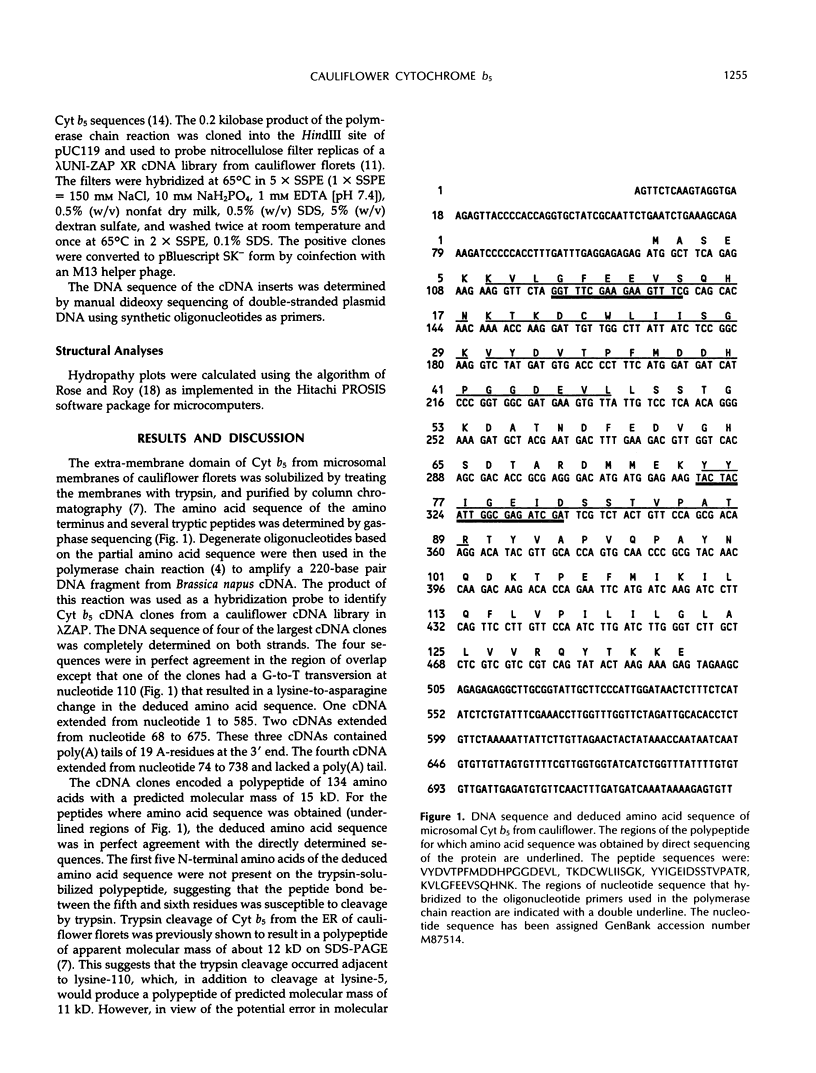
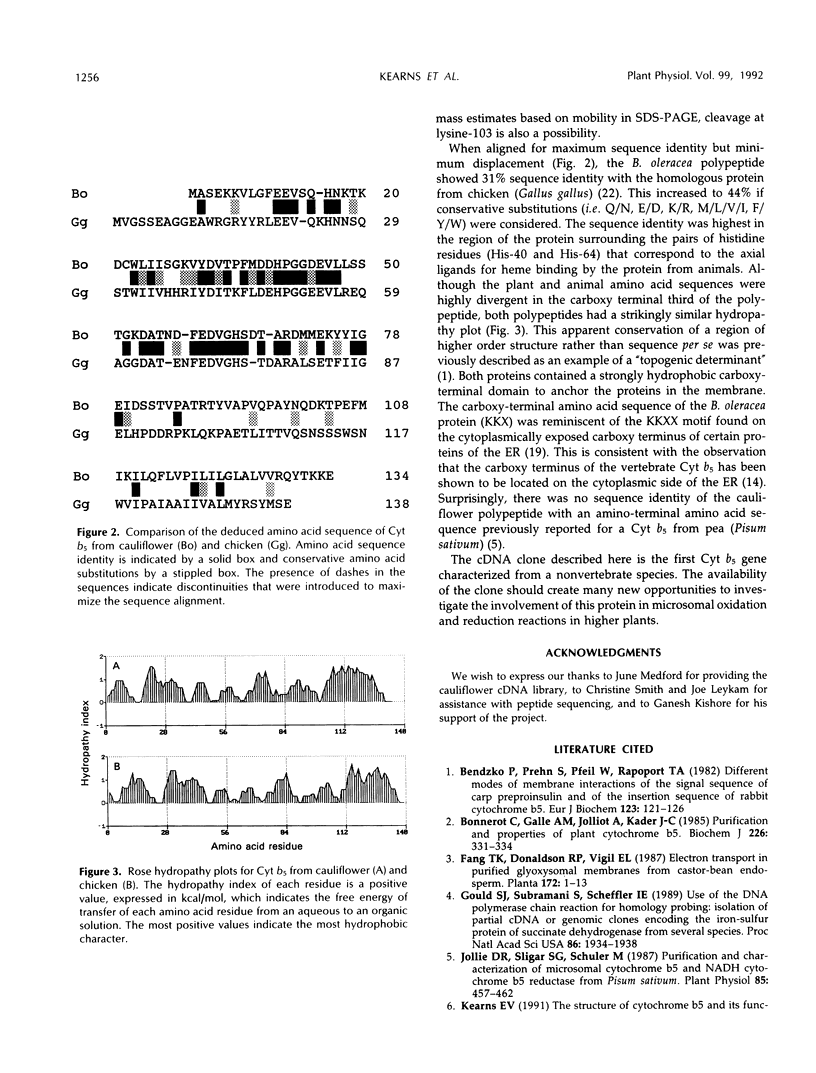
Cytochrome b5 is a microsomal protein that functions as an intermediate electron donor in fatty acid desaturation and other oxidation/reduction reactions. cDNA clones were isolated from cauliflower (Brassica oleracea L.) by using oligonucleotides based on the partial amino acid sequence of the protein. The deduced amino acid sequence of the polypeptide exhibited approximately 30% sequence identity with the homologous protein from vertebrates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendzko P., Prehn S., Pfeil W., Rapoport T. A. Different modes of membrane interactions of the signal sequence of carp preproinsulin and of the insertion sequence of rabbit cytochrome b5. Eur J Biochem. 1982 Mar;123(1):121–126. doi: 10.1111/j.1432-1033.1982.tb06507.x. [DOI] [PubMed] [Google Scholar]
- Bonnerot C., Galle A. M., Jolliot A., Kader J. C. Purification and properties of plant cytochrome b5. Biochem J. 1985 Feb 15;226(1):331–334. doi: 10.1042/bj2260331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Subramani S., Scheffler I. E. Use of the DNA polymerase chain reaction for homology probing: isolation of partial cDNA or genomic clones encoding the iron-sulfur protein of succinate dehydrogenase from several species. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1934–1938. doi: 10.1073/pnas.86.6.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollie D. R., Sligar S. G., Schuler M. Purification and Characterization of Microsomal Cytochrome b(5) and NADH Cytochrome b(5) Reductase from Pisum sativum. Plant Physiol. 1987 Oct;85(2):457–462. doi: 10.1104/pp.85.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns E. V., Hugly S., Somerville C. R. The role of cytochrome b5 in delta 12 desaturation of oleic acid by microsomes of safflower (Carthamus tinctorius L.). Arch Biochem Biophys. 1991 Feb 1;284(2):431–436. doi: 10.1016/0003-9861(91)90319-e. [DOI] [PubMed] [Google Scholar]
- Keyes S. R., Alfano J. A., Jansson I., Cinti D. L. Rat liver microsomal elongation of fatty acids. Possible involvement of cytochrome b5. J Biol Chem. 1979 Aug 25;254(16):7778–7784. [PubMed] [Google Scholar]
- Lee T. C., Baker R. C., Stephens N., Snyder F. Evidence for participation of cytochrome b5 in microsomal delta-6 desaturation of fatty acids. Biochim Biophys Acta. 1977 Oct 24;489(1):25–31. doi: 10.1016/0005-2760(77)90228-4. [DOI] [PubMed] [Google Scholar]
- Madyastha K. M., Krishnamachary N. Purification and partial characterization of microsomal cytochrome b555 from the higher plant Catharanthus roseus. Biochem Biophys Res Commun. 1986 Apr 29;136(2):570–576. doi: 10.1016/0006-291x(86)90478-x. [DOI] [PubMed] [Google Scholar]
- Medford J. I., Elmer J. S., Klee H. J. Molecular cloning and characterization of genes expressed in shoot apical meristems. Plant Cell. 1991 Apr;3(4):359–370. doi: 10.1105/tpc.3.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshiro M., Omura T. Immunochemical study on the electron pathway from NADH to cytochrome P-450 of liver microsomes. J Biochem. 1978 Jan;83(1):61–77. doi: 10.1093/oxfordjournals.jbchem.a131913. [DOI] [PubMed] [Google Scholar]
- Ozols J. Structure of cytochrome b5 and its topology in the microsomal membrane. Biochim Biophys Acta. 1989 Jul 27;997(1-2):121–130. doi: 10.1016/0167-4838(89)90143-x. [DOI] [PubMed] [Google Scholar]
- Paltuaf F., Prough R. A., Masters B. S., Johnston J. M. Evidence for the participation of cytochrome b5 in plasmalogen biosynthesis. J Biol Chem. 1974 Apr 25;249(8):2661–2662. [PubMed] [Google Scholar]
- Reddy V. V., Kupfer D., Caspi E. Mechanism of C-5 double bond introduction in the biosynthesis of cholesterol by rat liver microsomes. J Biol Chem. 1977 May 10;252(9):2797–2801. [PubMed] [Google Scholar]
- Rich P. R., Bendall D. S. Cytochrome components of plant microsomes. Eur J Biochem. 1975 Jul 1;55(2):333–341. doi: 10.1111/j.1432-1033.1975.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Roy S. Hydrophobic basis of packing in globular proteins. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4643–4647. doi: 10.1073/pnas.77.8.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Cross A. R., Jones O. T., Griffiths W. T., Stymne S., Stobart K. Electron-transport components of the 1-acyl-2-oleoyl-sn-glycero-3-phosphocholine delta 12-desaturase (delta 12-desaturase) in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons. Biochem J. 1990 Nov 15;272(1):23–29. doi: 10.1042/bj2720023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Spatz L., Corcoran D., Rogers M. J., Setlow B., Redline R. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4565–4569. doi: 10.1073/pnas.71.11.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Somerville C. Soluble and membrane-bound forms of cytochrome b5 are the products of a single gene in chicken. Arch Biochem Biophys. 1990 Aug 1;280(2):412–415. doi: 10.1016/0003-9861(90)90350-8. [DOI] [PubMed] [Google Scholar]


