Abstract
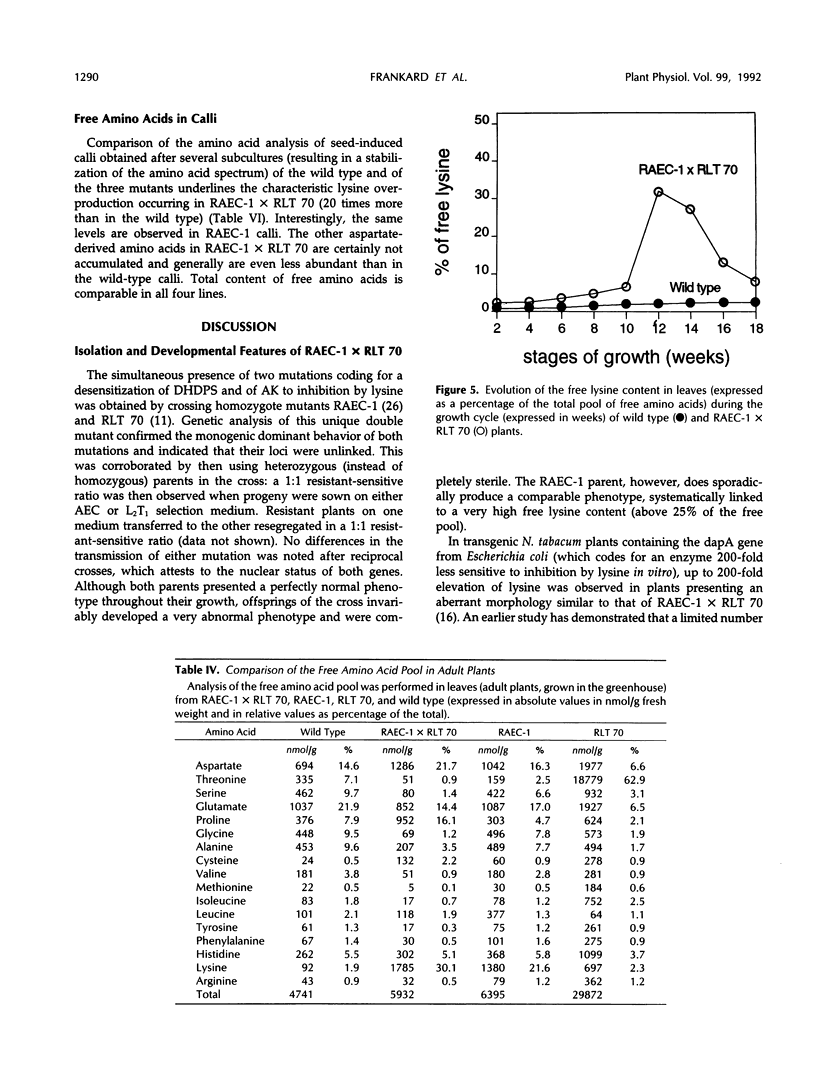
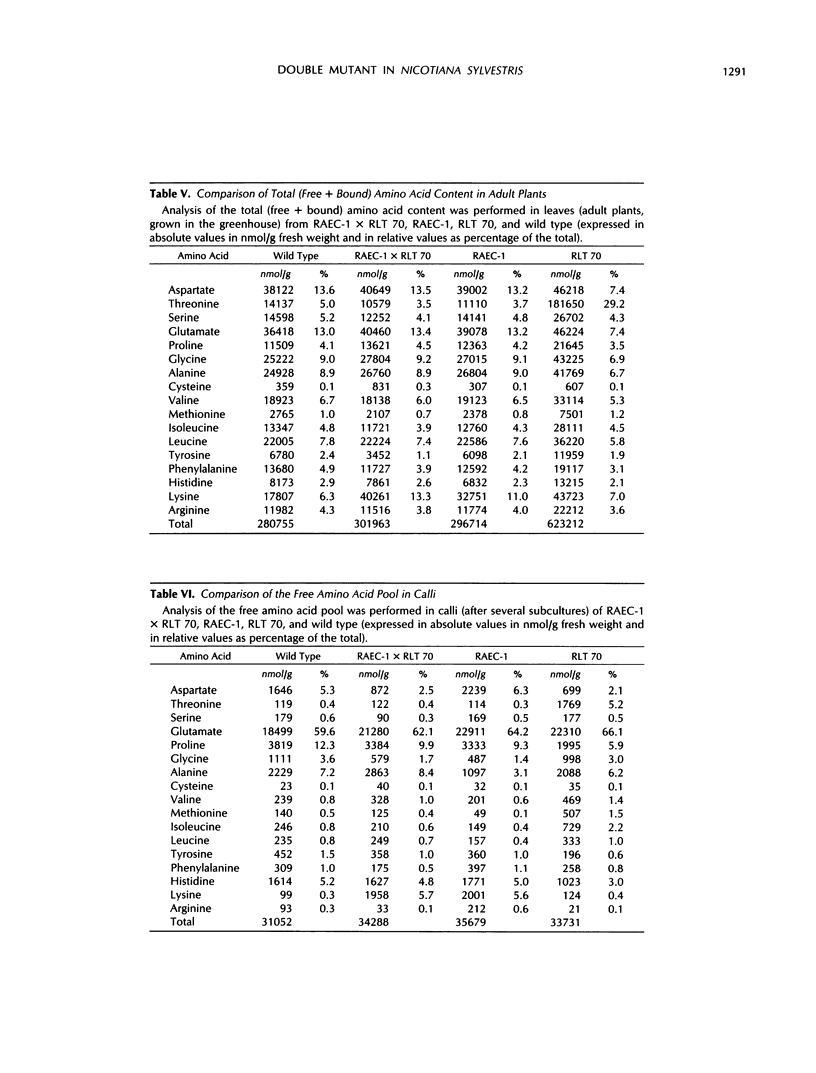
Lysine and threonine overproducer mutants in Nicotiana sylvestris, characterized by an altered regulation of, respectively, dihydrodipicolinate synthase and aspartate kinase activities, were crossed to assess the effects of the simultaneous presence of these genes on the biosynthesis of aspartate-derived amino acids. The monogenic dominant behavior of both resistance traits was confirmed, and their loci were found to be unlinked. Study of the inhibition properties of dihydrodipicolinate synthase and aspartate kinase activities in RAEC-1 × RLT 70 confirmed the heterozygote state of both mutations, because only half of their lysine-sensitive activity could still be inhibited by this negative effector. Analysis of the free amino acid pool during the growth of the double mutant revealed a major free lysine overproduction reaching up to 50% of the total pool, whereas the other aspartate-derived amino acids remained equally or even less abundant than in the wild type. An abnormal phenotype was clearly associated with such high levels of lysine accumulation, which points out the possible role of this amino acid in the developmental features of the plant. Comparison of the amino acid content, free and total (free + protein-bound), between the wild type, the two mutants, and the double mutant obtained by crossing them brings new insights on the regulation of the aspartate pathway, and on its implications in relationship to plant nutritional value improvement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieleski R. L., Turner N. A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966 Nov;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Bryan P. A., Cawley R. D., Brunner C. E., Bryan J. K. Isolation and characterization of a lysine-sensitive aspartokinase from a multicellular plant. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1211–1217. doi: 10.1016/0006-291x(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Fuller M. F., Mennie I., Crofts R. M. The amino acid supplementation of barley for the growing pig. 2. Optimal additions of lysine and threonine for growth. Br J Nutr. 1979 Mar;41(2):333–340. doi: 10.1079/bjn19790042. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Regulatory Structure of the Biosynthetic Pathway for the Aspartate Family of Amino Acids in Lemna paucicostata Hegelm. 6746, with Special Reference to the Role of Aspartokinase. Plant Physiol. 1989 Aug;90(4):1584–1599. doi: 10.1104/pp.90.4.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd K. A., Green C. E. Inheritance and expression of lysine plus threonine resistance selected in maize tissue culture. Proc Natl Acad Sci U S A. 1982 Jan;79(2):559–563. doi: 10.1073/pnas.79.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer G. W., Sharpe F. T. Increased Lysine and Seed Storage Protein in Rice Plants Recovered from Calli Selected with Inhibitory Levels of Lysine plus Threonine and S-(2-Aminoethyl)cysteine. Plant Physiol. 1987 Jun;84(2):509–515. doi: 10.1104/pp.84.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]