Abstract
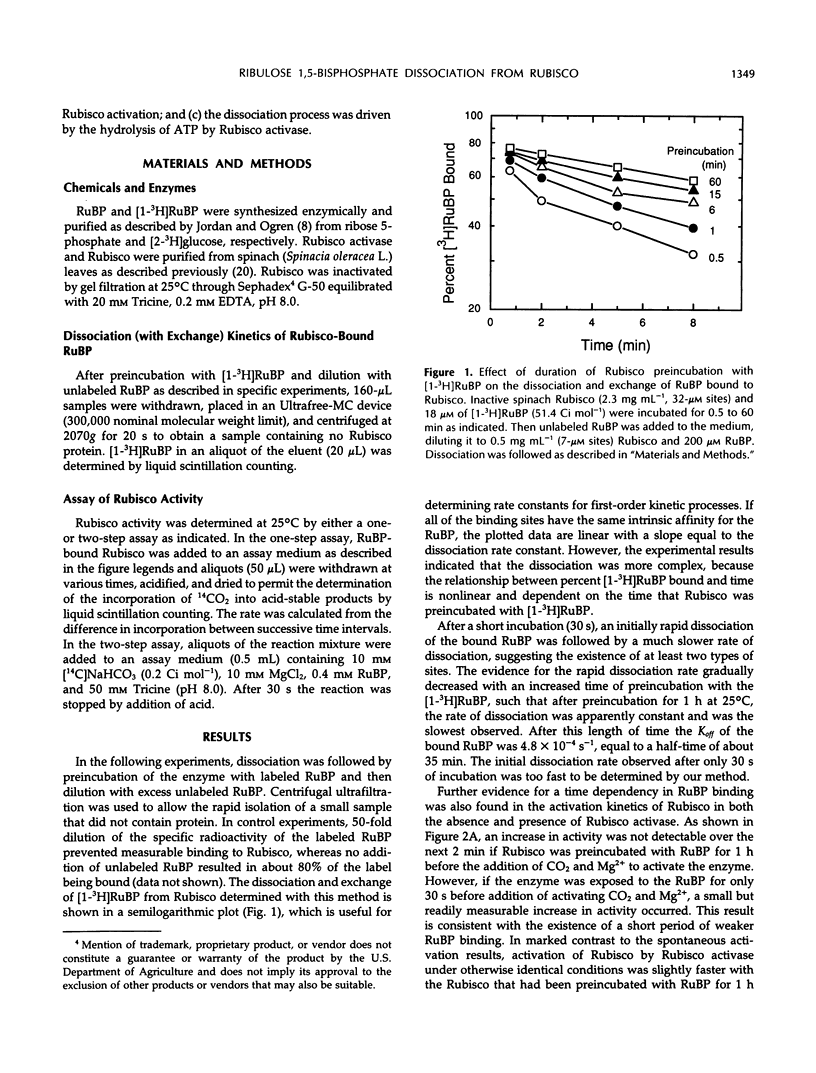
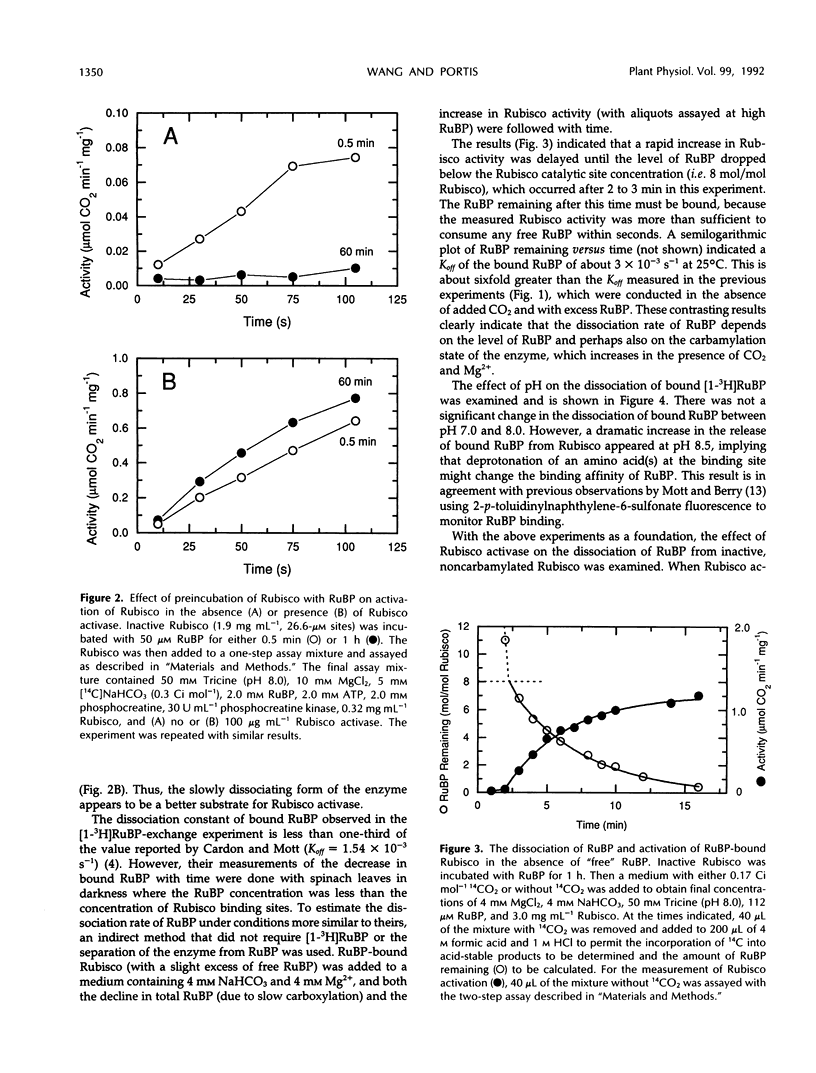
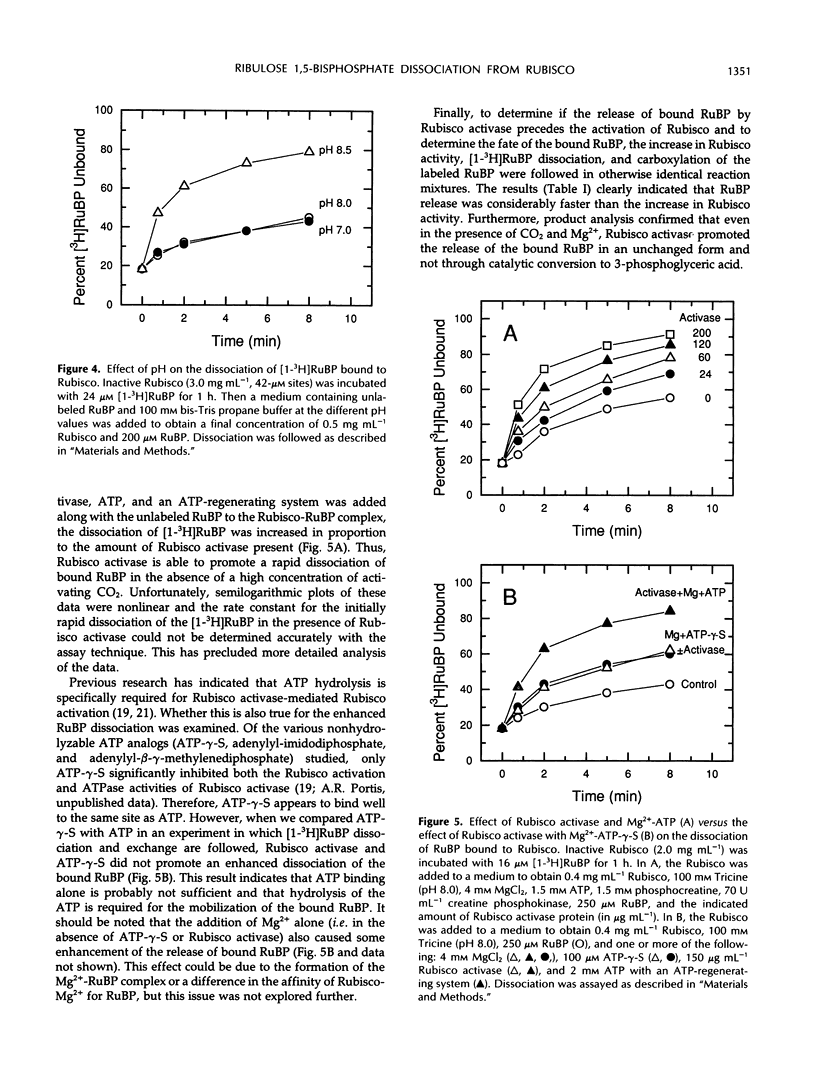
Ribulose bisphosphate (RuBP), a substrate of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), is an inhibitor of Rubisco activation by carbamylation if bound to the inactive, noncarbamylated form of the enzyme. The effect of Rubisco activase on the dissociation kinetics of RuBP bound to this form of the enzyme was examined and characterized with the use of 3H-labeled RuBP and proteins purified from spinach (Spinacia oleracea L.) In the absence of Rubisco activase and in the presence of a large excess of unlabeled RuBP, the dissociation rate of bound [1-3H]RuBP was much faster after a short (30 second) incubation than after an extended incubation (1 hour). After 1 hour of incubation, the dissociation rate constant (Koff) of the bound RuBP was 4.8 × 10−4 per second, equal to a half-time of about 35 minutes, whereas the rate after only 30 seconds was too fast to be accurately measured. This time-dependent change in the dissociation rate was reflected in the subsequent activation kinetics of Rubisco in the presence of RuBP, CO2, and Mg2+, and in both the absence or presence of Rubisco activase. However, the activation of Rubisco also proceeded relatively rapidly without Rubisco activase if the RuBP level decreased below the estimated catalytic site concentration. High pH (pH 8.5) and the presence of Mg2+ in the medium also enhanced the dissociation of the bound RuBP from Rubisco in the presence of RuBP. In the presence of Rubisco activase, Mg2+, ATP (but not the nonhydrolyzable analog, adenosine-5′-O-[3-thiotriphosphate]), excess RuBP, and an ATP-regenerating system, the dissociation of [1-3H]RuBP from Rubisco was increased in proportion to the amount of Rubisco activase added. This result indicates that Rubisco activase-mediated hydrolysis of ATP is required for promotion of the enhanced dissociation of the bound RuBP from Rubisco. Furthermore, product analysis by ion-exchange chromatography demonstrated that the release of the bound RuBP, in an unchanged form, was considerably faster than the observed increase in Rubisco activity. Thus, RuBP dissociation was experimentally separated from activation and precedes the subsequent formation of active, carbamylated Rubisco during activation of Rubisco by Rubisco activase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardon Z. G., Mott K. A. Evidence that Ribulose 1,5-Bisphosphate (RuBP) Binds to Inactive Sites of RuBP Carboxylase in Vivo and an Estimate of the Rate Constant for Dissociation. Plant Physiol. 1989 Apr;89(4):1253–1257. doi: 10.1104/pp.89.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. L., Badger M. R., Andrews T. J. Slow Inactivation of Ribulosebisphosphate Carboxylase during Catalysis Is Not Due to Decarbamylation of the Catalytic Site. Plant Physiol. 1990 Aug;93(4):1383–1389. doi: 10.1104/pp.93.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. B., Chollet R. Inhibition of ribulose bisphosphate carboxylase by substrate ribulose 1,5-bisphosphate. J Biol Chem. 1983 Nov 25;258(22):13752–13758. [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981 Feb;67(2):237–245. doi: 10.1104/pp.67.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Portis A. R. Activation of ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) by rubisco activase : effects of some sugar phosphates. Plant Physiol. 1990 Sep;94(1):245–250. doi: 10.1104/pp.94.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Mott K. A., Berry J. A. Effects of pH on Activity and Activation of Ribulose 1,5-Bisphosphate Carboxylase at Air Level CO(2). Plant Physiol. 1986 Sep;82(1):77–82. doi: 10.1104/pp.82.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Salvucci M. E., Ogren W. L. Activation of Ribulosebisphosphate Carboxylase/Oxygenase at Physiological CO(2) and Ribulosebisphosphate Concentrations by Rubisco Activase. Plant Physiol. 1986 Dec;82(4):967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R., Jr Adenosine triphosphate hydrolysis by purified rubisco activase. Arch Biochem Biophys. 1989 Jan;268(1):93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein prevents the in vitro decline in activity of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1989 Jul;90(3):968–971. doi: 10.1104/pp.90.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Streusand V. J., Chatfield J. M., Portis A. R. Purification and assay of rubisco activase from leaves. Plant Physiol. 1988 Dec;88(4):1008–1014. doi: 10.1104/pp.88.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streusand V. J., Portis A. R. Rubisco Activase Mediates ATP-Dependent Activation of Ribulose Bisphosphate Carboxylase. Plant Physiol. 1987 Sep;85(1):152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolieres A., Darmency H., Gasquez J., Dron M., Connan A. Variation of Transhexadecenoic Acid Content in Two Triazine Resistant Mutants of Chenopodium album and Their Susceptible Progenitor. Plant Physiol. 1988 Mar;86(3):967–970. doi: 10.1104/pp.86.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Chatfield J. M., Ogren W. L. Catalysis of Ribulosebisphosphate Carboxylase/Oxygenase Activation by the Product of a Rubisco Activase cDNA Clone Expressed in Escherichia coli. Plant Physiol. 1988 Aug;87(4):917–920. doi: 10.1104/pp.87.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]


