Abstract
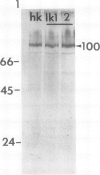
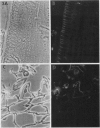
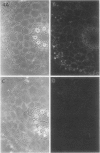
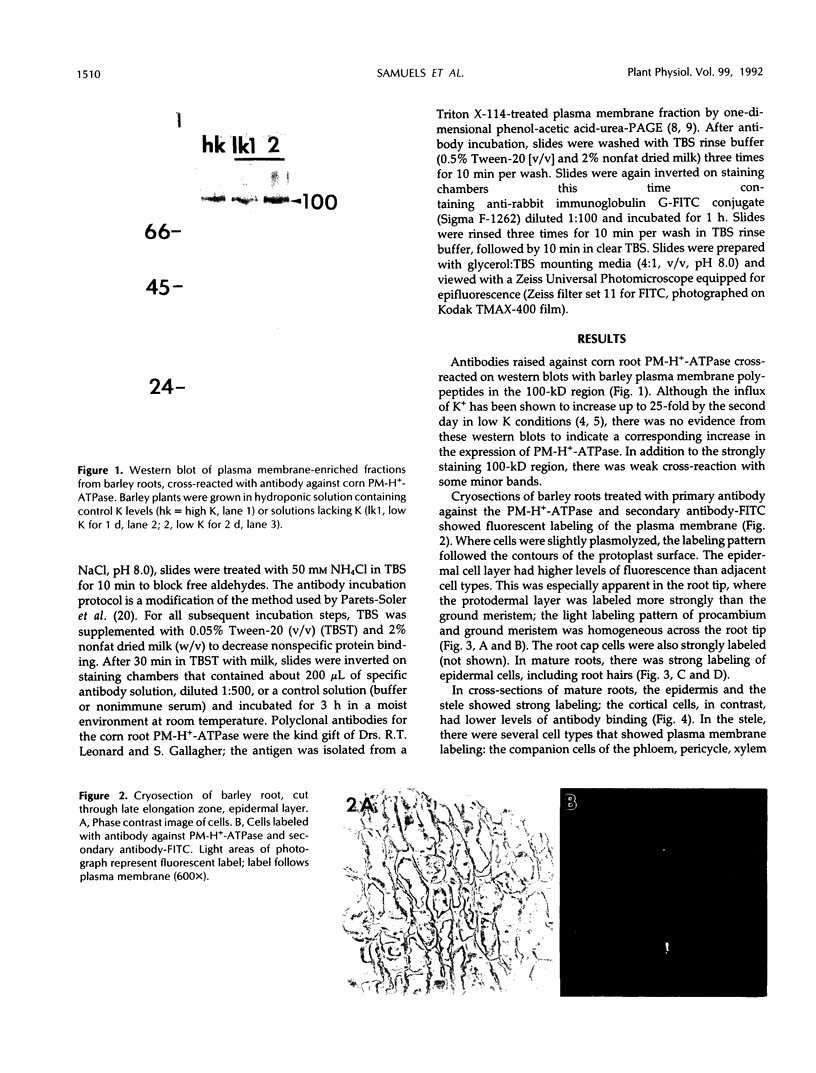
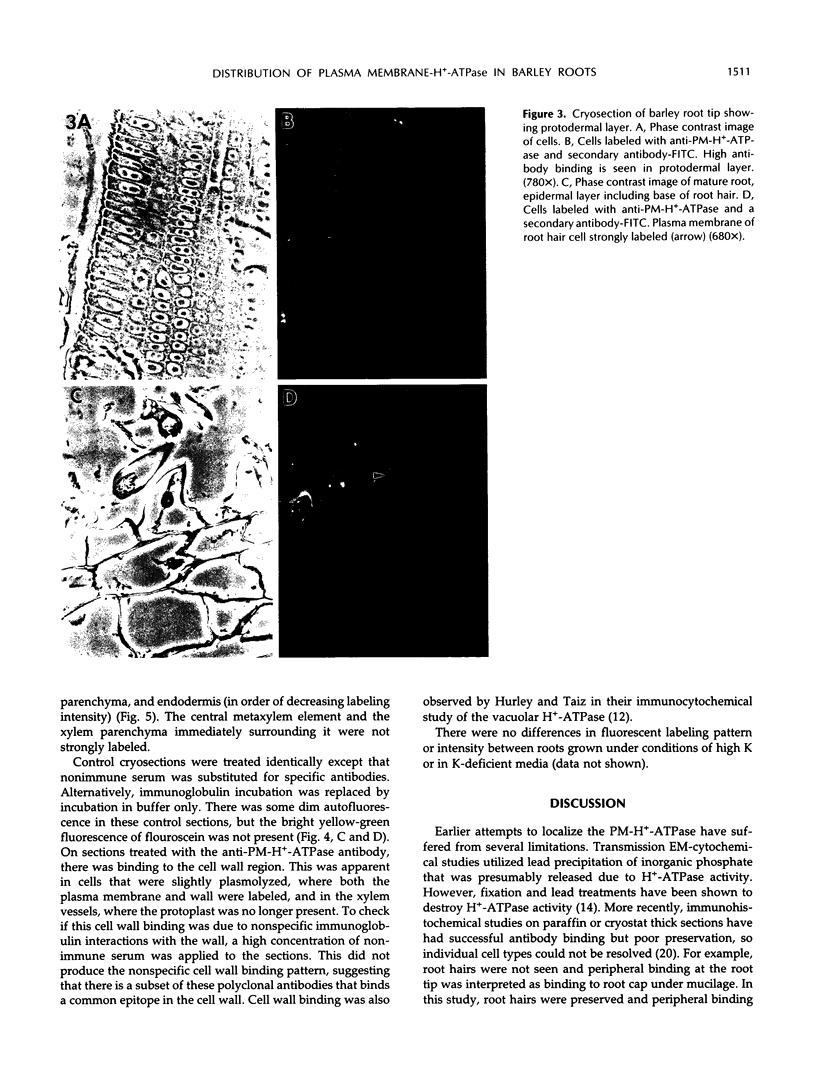
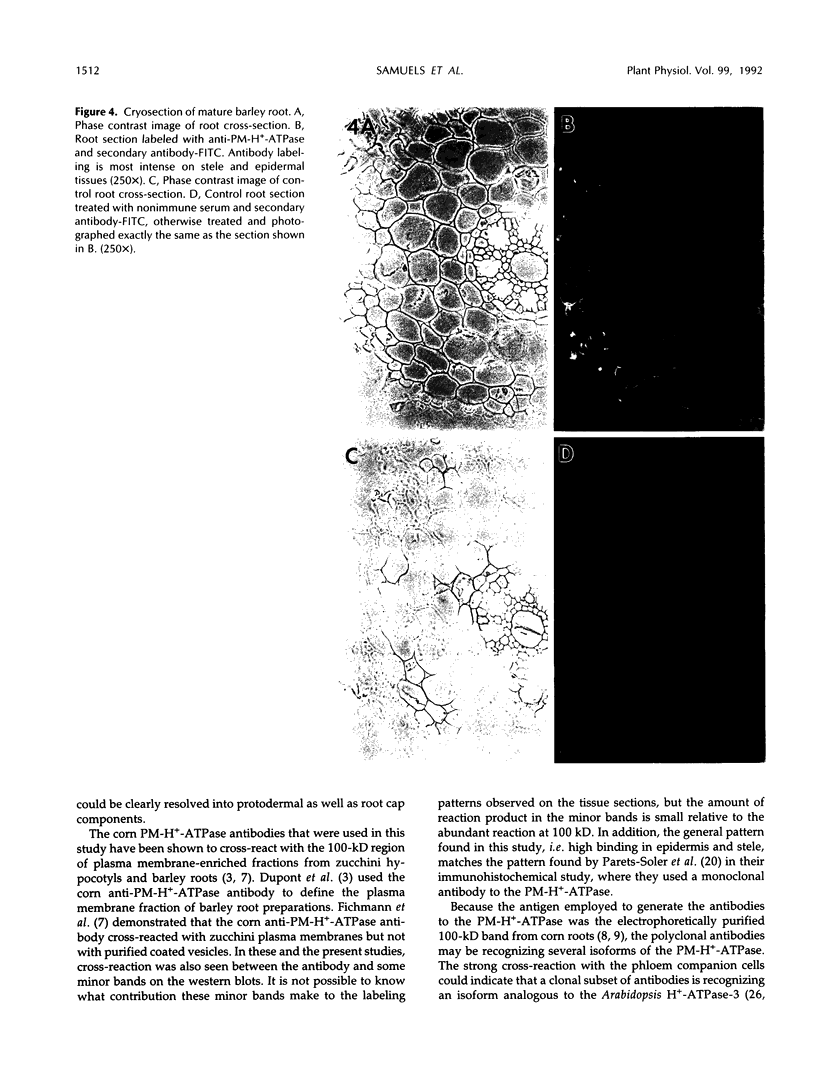
The plasma membrane H+-ATPase (PM-H+-ATPase) of barley (Hordeum vulgare L. cv Klondike) roots was assayed by cross-reaction on western blots and cryosections with an antibody against the PM-H+-ATPase from corn roots. Under conditions of reduced K availability, which have previously been shown to increase K influx by greater than 25-fold, there were only minor changes detected in PM-H+-ATPase levels. Antibody labeling of cryosections showed the relative distribution of PM-H+-ATPase among cell types in root tips and mature roots. Epidermal cells, both protoderm and mature root epidermis, including root hairs, had high levels of antibody binding. In mature roots, the stelar tissue showing the highest antibody binding was the companion cells of the phloem, followed by pericycle, xylem parenchyma, and endodermis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dupont F. M., Tanaka C. K., Hurkman W. J. separation and Immunological Characterization of Membrane Fractions from Barley Roots. Plant Physiol. 1988 Mar;86(3):717–724. doi: 10.1104/pp.86.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando M., Kulpa J., Siddiqi M. Y., Glass A. D. Potassium-dependent changes in the expression of membrane-associated proteins in barley roots : I. Correlations with k(rb) influx and root k concentration. Plant Physiol. 1990 Apr;92(4):1128–1132. doi: 10.1104/pp.92.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Electrophoretic characterization of a detergent-treated plasma membrane fraction from corn roots. Plant Physiol. 1987 Feb;83(2):265–271. doi: 10.1104/pp.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. B., Sussman M. R., Mierzwa R. J., Evert R. F. Cytochemical localization of ATPase activity in oat roots localizes a plasma membrane-associated soluble phosphatase, not the proton pump. Plant Physiol. 1988 Mar;86(3):841–847. doi: 10.1104/pp.86.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Parets-Soler A., Pardo J. M., Serrano R. Immunocytolocalization of Plasma Membrane H-ATPase. Plant Physiol. 1990 Aug;93(4):1654–1658. doi: 10.1104/pp.93.4.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia T., Poole R. J. ATP Levels and their Effects on Plasmalemma Influxes of Potassium Chloride in Red Beet. Plant Physiol. 1980 May;65(5):969–972. doi: 10.1104/pp.65.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Transport across plant roots. Q Rev Biophys. 1982 Aug;15(3):481–554. doi: 10.1017/s0033583500003437. [DOI] [PubMed] [Google Scholar]
- Sussman M. R., Harper J. F. Molecular biology of the plasma membrane of higher plants. Plant Cell. 1989 Oct;1(10):953–960. doi: 10.1105/tpc.1.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- White M. J., Green B. R. Antibodies to the photosystem I chlorophyll a + b antenna cross-react with polypeptides of CP29 and LHCII. Eur J Biochem. 1987 Mar 16;163(3):545–551. doi: 10.1111/j.1432-1033.1987.tb10902.x. [DOI] [PubMed] [Google Scholar]