Abstract
Alanine aminotransferase (AlaAT, EC 2.6.1.2) is an enzyme that is induced under anaerobic conditions in cereal roots. In barley (Hordeum vulgare L.) roots, there are a number of isoforms of AlaAT. We have identified the anaerobically induced isoform and have purified it to homogeneity. The isolation procedure involved a two-step ammonium sulfate precipitation, gel filtration, ion-exchange chromatography, and chromatofocusing. The enzyme was purified approximately 350-fold to a specific activity of 2231 units/milligram protein. The apparent molecular masses of the native and sodium dodecyl sulfate-denatured AlaAT proteins are 97 and 50 kilodaltons, respectively, indicating that the native enzyme is probably a homodimer. AlaAT has a number of interesting characteristics when compared with other plant aminotransferases. AlaAT does not require the presence of pyridoxyl-5-phosphate to retain its activity, and it appears to be very specific in the reactions that it will catalyze.
Full text
PDF

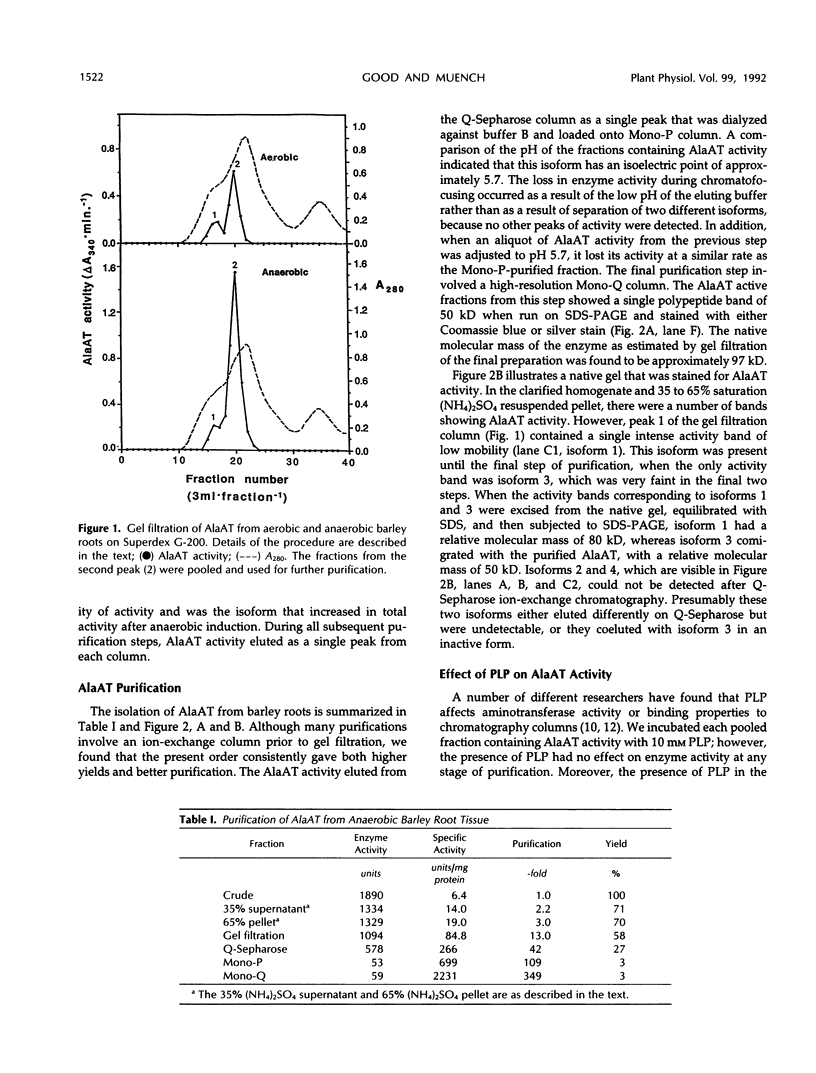
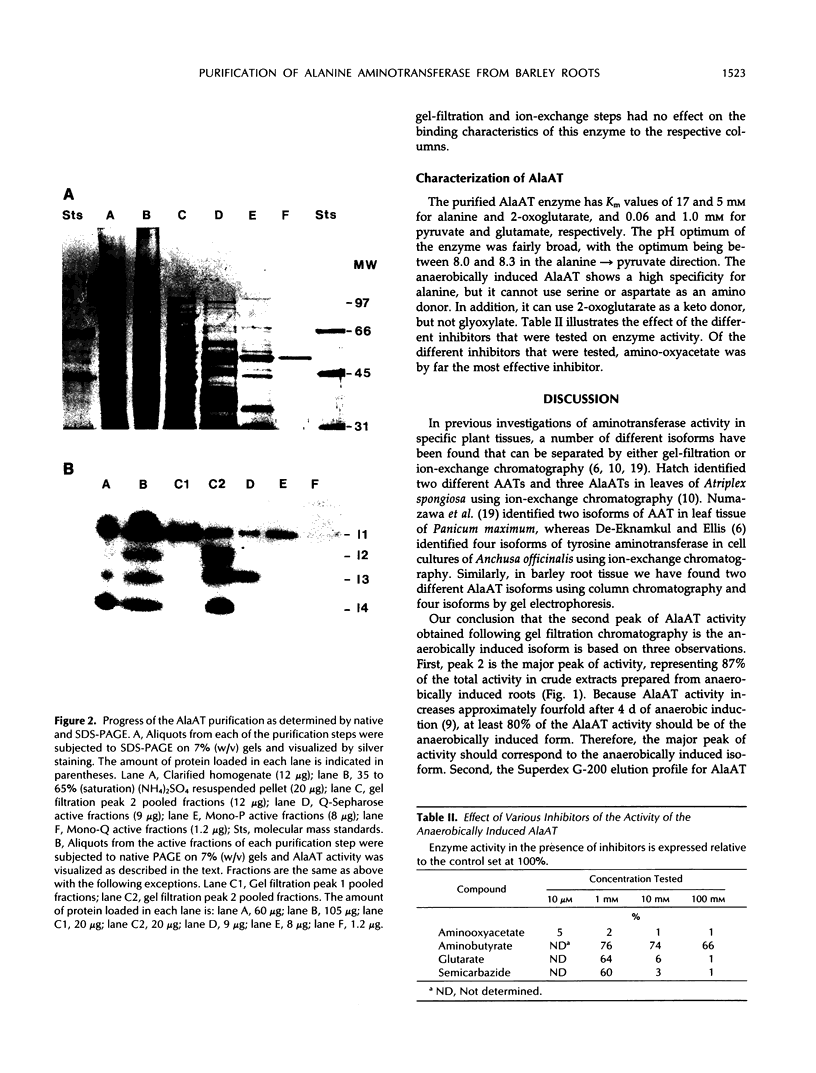


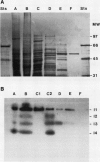
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULOS B., HANDLER P. KINETICS OF BEEF HEART GLUTAMIC-ALANINE TRANSAMINASE. J Biol Chem. 1965 Aug;240:3283–3294. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- De-Eknamkul W., Ellis B. E. Purification and characterization of tyrosine aminotransferase activities from Anchusa officinalis cell cultures. Arch Biochem Biophys. 1987 Sep;257(2):430–438. doi: 10.1016/0003-9861(87)90587-x. [DOI] [PubMed] [Google Scholar]
- Effer W. R., Ranson S. L. Some effects of oxygen concentration on levels of respiratory intermediates in buckwheat seedlings. Plant Physiol. 1967 Aug;42(8):1053–1058. doi: 10.1104/pp.42.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good A. G., Crosby W. L. Anaerobic induction of alanine aminotransferase in barley root tissue. Plant Physiol. 1989 Aug;90(4):1305–1309. doi: 10.1104/pp.90.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good A. G., Crosby W. L. Induction of alcohol dehydrogenase and lactate dehydrogenase in hypoxically induced barley. Plant Physiol. 1989 Jul;90(3):860–866. doi: 10.1104/pp.90.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Vose B. M., Chu E. W., Wilson D. J., Lotze M. T., Rayner A. A., Rosenberg S. A. The human mixed lymphocyte-tumor cell interaction test. I. Positive autologous lymphocyte proliferative responses can be stimulated by tumor cells as well as by cells from normal tissues. Cancer Immunol Immunother. 1984;17(2):83–89. doi: 10.1007/BF00200041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. Separation and properties of leaf aspartate aminotransferase and alanine aminotransferase isoenzymes operative in the C4 pathway of photosynthesis. Arch Biochem Biophys. 1973 May;156(1):207–214. doi: 10.1016/0003-9861(73)90358-5. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Bent A. F., Hanson A. D. Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol. 1986 Nov;82(3):658–663. doi: 10.1104/pp.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondred D., Hunter J. M., Keith R., Titus D. E., Becker W. M. Isolation of serine:glyoxylate aminotransferase from cucumber cotyledons. Plant Physiol. 1985 Sep;79(1):95–102. doi: 10.1104/pp.79.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J Biol Chem. 1984 Nov 25;259(22):14180–14183. [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize glucose phosphate isomerase I. J Biol Chem. 1984 Jan 10;259(1):673–677. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Tolbert N. E. Serine: glyoxylate, alanine:glyoxylate, and glutamate:glyoxylate aminotransferase reactions in peroxisomes from spinach leaves. J Biol Chem. 1983 Jun 25;258(12):7631–7638. [PubMed] [Google Scholar]
- Numazawa T., Yamada S., Hase T., Sugiyama T. Aspartate aminotransferase from Panicum maximum Jacq. var. trichoglume Eyles, a C4 plant: purification, molecular properties, and preparation of antibody. Arch Biochem Biophys. 1989 Apr;270(1):313–319. doi: 10.1016/0003-9861(89)90033-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. Characteristics of alanine: glyoxylate aminotransferase from Saccharomyces cerevisiae, a regulatory enzyme in the glyoxylate pathway of glycine and serine biosynthesis from tricarboxylic acid-cycle intermediates. Biochem J. 1985 Oct 1;231(1):157–163. doi: 10.1042/bj2310157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe G. C., Feil R., Turpin D. H. Anaerobic Metabolism in the N-Limited Green Alga Selenastrum minutum: I. Regulation of Carbon Metabolism and Succinate as a Fermentation Product. Plant Physiol. 1990 Nov;94(3):1116–1123. doi: 10.1104/pp.94.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]