Abstract
Background
Small for gestational age (SGA) poses a significant concern for newborns, being linked to neonatal complications and potential metabolic disorders in adulthood, especially when born to mothers with gestational diabetes mellitus (GDM), elevating their risk of complications and mortality. However, the pregnancy risk factors and glycaemic control associated with SGA infants born to mothers with GDM remain unclear.
Aim
To identify the pregnancy risk factors and glycaemic control associated with SGA infants born to mothers with GDM.
Method
This case–control study was conducted among 1910 women with GDM in China. Data were collected by the integrated electronic medical record system. Using 1:4 propensity score matching analysis, we adjusted for gestational age as confounder. Univariate and multivariate analyses were performed to identify risk factors.
Results
Risk factors for SGA born to mothers with GDM included a history of low birth weight, gestational hypertension, oligohydramnios, short maternal height, underweight pre-pregnancy body mass index and inadequate weight growth. While SGA was protected by weakly positive ketonuria levels in the first trimester, multiparous, anaemia and previous uterine scar were protective factors for SGA. Moreover, 2-hour postprandial glucose and haemoglobin A1c in the second trimester, as well as the 0-hour and 2-hour 75 g oral glucose tolerance test (OGTT) were linked to risk of SGA.
Conclusions
SGA infants are the result of multifactorial interactions among GDM pregnant women. Notably, glycaemic control levels were associated with SGA. There is a need for enhanced perinatal monitoring and antenatal care to reduce SGA.
Keywords: Risk Factors, Diabetes in pregnancy, Child protection
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Propensity score matching effectively controlled for confounding variables and reduced bias, enhancing the study’s result validity. This approach provided credible insights into risk factors and glycaemic control for small-for-gestational-age infants born to mothers with gestational diabetes mellitus.
A large population size increases statistical power, enabling the detection of subtle associations and providing more generalisable findings.
As a case–control study relying on retrospective data from medical records, there might be limitations the incorporation of subjective information.
The findings may primarily apply to the specific population from which the data was collected, limiting their generalisability to other regions or diverse populations.
Background
Gestational diabetes mellitus (GDM) is a glucose intolerance that develops or first becomes detectable during pregnancy,1 which has the most common metabolic disease and affected up to 25% of pregnant women.2 In China, the prevalence of GDM has been increasing, with 14.8% of pregnant women now affected.3 This condition gives rise to a range of short-term and long-term maternal and fetal health issues, particularly associated with increased pace of fetal growth. Fetuses receive increased amounts of glucose through maternal hyperglycaemia, which promotes insulin secretion and increases fetal growth.4 Furthermore, hyperglycaemia causes placental vascular dysfunction, reducing the supply of oxygen and nutrients to the fetus.5 There is still 2.7% GDM pregnant women who deliver children that experience fetal growth restrictions (FGRs).6 Additionally, the incidence of small-for-gestational-age (SGA) infants born to mothers with GDM was 6.45% in China.7 However, limited research is available on SGA infants born to Chinese women with GDM.
SGA infants are commonly defined as having birth weight below the 10th percentile for a given gestational age and sex,8 including infants who are naturally small without pathological growth restriction. In China, the total number of SGA births is the fifth highest in the world,9 imposing a tremendous medical and socioeconomic burden. SGA infants have an increased risk of adverse perinatal outcomes, such as stillbirth, asphyxia or birth defects. Additionally, compared with appropriate-for-gestational-age (AGA) infants, SGA infants are prone to have poor cognitive or psychological outcomes as well as metabolic diseases, such as type 2 diabetes, insulin resistance and arterial hypertension in adulthood.10 11 In addition, GDM has been linked to delayed development and stunted fetal growth.12 This linkage may exacerbate the adverse health outcomes of SGA. Epidemiological studies show that SGA infants born to mothers with GDM have higher rates of neonatal complications or death.13 14 They are also at higher risk of developing long-term cardiovascular offspring hospitalisation.15 Given the seriousness of the consequences, identifying its potential influencing factors is of great significance for the screening and prevention of SGA births among GDM pregnant women.
Maternal glycaemia is widely recognised for its association with perinatal outcomes, including its impact on offspring birth weight.16 According to Hyperglycaemia and Adverse Pregnancy Outcome (HAPO), women with higher glucose levels are considered to be at greater risk.17 Current prenatal treatment goals emphasise tight glucose monitoring and strict glucose control.18 19 Consequently, women experiencing hypoglycaemia are generally deemed to be at low risk for antenatal care. Several investigations have reported an association between maternal hypoglycaemia and FGR or SGA.20–23 Presently, the pregnancy factors related to SGA infants born to women with GDM remain unclear. Moreover, few studies have examined the association between maternal glycaemic level associated with SGA infants born to mothers with GDM. After the diagnosis of GDM, timely recognition of glycaemic abnormalities is critical for normal fetal growth and development. Therefore, the purpose of this study was to explore the influencing factors and glycaemic control during pregnancy associated with SGA infants born to mothers with GDM in China.
Methods
Study design and population
This case–control study included pregnancies affected by GDM who delivered between January 2019 and December 2020 from a tertiary Maternal and Child Health Hospital in Fuzhou City, Fujian Province. All pregnant women followed a routine prenatal care protocol, scheduling frequent visits to the health system to identification of risk factors and initiation of preventive care measures.24
Eligible participants were pregnant women diagnosed with GDM based on 75 g oral glucose tolerance test (OGTT) conducted between 24 and 32 weeks of gestation, following the modified International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria.25 Diagnostic criteria included one or more elevated glucose levels: fasting plasma glucose (FPG) level ≥5.1 mmol/L, 1-hour plasma glucose level ≥10.0 mmol/L and 2-hour plasma glucose level ≥8.5 mmol/L.25 Pregnant women with multiple gestations, a clinical diagnosis of pregestational diabetes mellitus or overt diabetes (FPG ≥7.0 mmol/L or 2 hours ≥11.0 mmol/L) were excluded. A total of 6839 participants were enrolled, all of whom had complete demographic and clinical data.
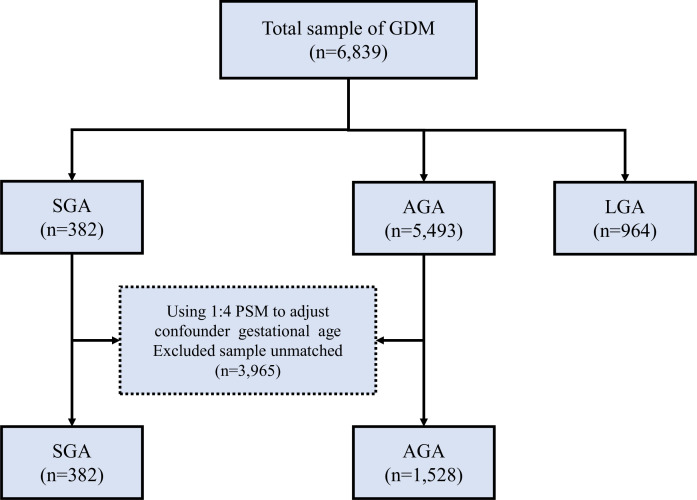
All participants in this study were categorised into the SGA group (case group, <10th percentile), AGA group (controlling group, between 10th and 90th percentile) and large-for-gestational-age (LGA) group (>90th percentile) according to the association between gestational age and birth weight. Finally, for each SGA infant, four gestational age-matched AGA infants were randomly selected using propensity score matching (PSM) analysis (figure 1).
Figure 1.
Flow diagram of selection of GDM pregnant women in this study. AGA, appropriate for gestational age; GDM, gestational diabetes mellitus; LGA, large for gestational age; PSM, propensity score matching; SGA, small for gestational age.
Patient and public involvement
No patients involved.
Data collection and study outcomes
Maternal demographic characteristics, pregnancy characteristics, pregnancy complications and outcomes were collected retrospectively by one researcher from the electronic medical record database of one hospital in our study. In addition, we collected glycaemic levels including 75 g OGTT glycaemia, FPG, 2-hour postprandial glucose and haemoglobin A1c (HbA1c) in the second trimester. Based on the number of abnormal OGTT values, women with GDM were stratified into 1, 2 or 3 items of abnormal OGTT values, respectively (online supplemental material 1).
bmjopen-2023-078325supp001.pdf (124.4KB, pdf)
The primary outcome of this study was SGA infants born to women with GDM. Gestational age was determined by subtracting the date of last menstrual period reported by the mother or by the first ultrasound scan from the date of birth. SGA was defined as birth weight below the 10th percentile for gestational age and sex, based on birth weight curves in China.26 27
Statistical analysis
All statistical analyses were performed using IBM SPSS, V.27.0, and R, V.4.1.3. We applied a 1:4 nearest-neighbour matching with a calliper of 0.01, a preset value for PSM, to lessen the potential selection bias and obtain matched data. The outcomes were compared between the SGA group and the AGA group among GDM pregnant women. Continuous variables were presented as mean ± SD and compared by using independent t-test. Categorical variables were presented as the frequency with percentages and analysed by the χ2 test or Fisher’s exact test.
We examined the risk factors associated with SGA infants born to mothers with GDM using the binary logistic regression model. Variables were carefully chosen to ensure parsimony of the final model (forward LR, entry 0.05, removal 0.10). Further, we investigated the association between maternal glycaemic levels and SGA, adjusted for various factors, including parity, previous uterine scar, history of low birth weight, history of abortion or miscarriage, history of macrosomia, gestational hypertensive disorder (HD), oligohydramnios, anaemia, pre-pregnancy BMI, height, gestational weight gain (GWG) rate and ketonuria in first trimester. A two-sided p value of <0.05 was considered statistically significant in all analyses.
Results
Selection of GDM pregnant women
A total of 6839 GDM pregnant women were enrolled in the study according to eligible and exclusion criteria, including 382 SGA infants, 964 LGA infants and 5493 AGA infants. After the 1:4 PSM analysis, 382 SGA infants were selected and 1528 AGA infants were randomly matched with the SGA group according to the gestational age at birth (figure 1). After propensity analysis, the mean (SD) gestational age at birth was 38.6 (SD=1.61) weeks in the AGA group and 38.59 (SD=1.62) weeks in the SGA group; there was no evidence of differences in the gestational age between the two groups (p=0.983).
Characteristics and univariate analysis of AGA and SGA
The average age of the participants was 31.67 (SD= 4.36) years old. Among all women, Han Chinese accounts for 97.91%. Approximately 50% of the participants in both groups had a college or university education. More than 50% of the women in the SGA group were nulliparous, which was slightly more than the percentage of women in the AGA group (35.3%) who were nulliparous (p<0.001). The previous uterine scar was shown to be statistically significant (p<0.001).
In terms of pregnancy history, there were no statistically significant differences observed in preterm delivery, fetal distress or GDM. However, a statistically significant association was found between a history of abortion or miscarriage (p=0.041), macrosomia (p=0.012) and low birth weight (p=0.011). Regarding pregnancy complications, statistically significant differences were identified in the occurrence of oligohydramnios (p<0.001) and anaemia (p=0.034). In addition, height, pre-pregnancy BMI and GWG rate were shown to be statistically significant (all p<0.05). Regarding the glycaemic laboratory parameters, 75 g OGTT 0-hour and 2-hour glycaemia, as well as ketonuria in first trimester, fasting glucose and 2-hour postprandial glucose in the second trimester showed statistical significance (p<0.05). However, 75 g OGTT 1 hour and HbA1c in the second trimester did not exhibit significant differences (p>0.05). The characteristics of the SGA group and AGA group are presented in tables 1 and 2 .
Table 1.
Maternal demographic characteristic of AGA group and SGA group matched according to 1:4 PSM analysis
| Variables | Items | AGA group(n=1528) | SGA group(n=382) | x2 | P value |
| Maternal age | 18~35 | 1220 (79.8) | 305 (79.8) | 0.395 | 1.000b |
| 36~45 | 305 (20) | 76 (19.9) | |||
| ≥46 | 3 (0.2) | 1 (0.3) | |||
| Nationality | The Han | 1498 (98) | 372 (97.4) | 0.638 | 0.424 |
| Minority nationality | 30 (2) | 10 (2.6) | |||
| Residence | Urban | 825 (54) | 196 (51.3) | 0.884 | 0.347 |
| Rural | 703 (46) | 186 (48.7) | |||
| Education | Elementary and below | 528 (34.6) | 126 (33) | 3.476 | 0.324 |
| Secondary/high school | 223 (14.6) | 45 (11.8) | |||
| College/university | 770 (50.4) | 210 (55) | |||
| Postgraduate or above | 7 (0.5) | 1 (0.3) | |||
| Occupation | Manual worker | 284 (18.6) | 69 (18.1) | 2.074 | 0.557 |
| Mental worker | 708 (46.3) | 192 (50.3) | |||
| Unemployed | 381 (24.9) | 86 (22.5) | |||
| Freelance | 155 (10.1) | 35 (9.2) | |||
| Marital status | Unmarried | 27 (1.8) | 8 (2.1) | 0.685 | 0.730b |
| Married | 1497 (98) | 374 (97.9) | |||
| Divorced or widowed | 4 (0.3) | 0 (0) | |||
| Height (cm) | ≥155 | 1248 (81.7) | 275 (72) | 22.232 | <0.001 |
| 150–154.9 | 197 (12.9) | 73 (19.1) | |||
| 145–149.9 | 79 (5.2) | 29 (7.6) | |||
| <145 | 4 (0.3) | 5 (1.3) | |||
| Pre-pregnancy BMI (kg/m2) | Normal | 1130 (74) | 271 (70.9) | 9.175 | 0.01 |
| Underweight | 172 (11.3) | 64 (16.8) | |||
| Overweight/obese | 226 (14.8) | 47 (12.3) | |||
| GWG rate | Inadequate gain | 690 (45.2) | 199 (52.1) | 6.107 | 0.047 |
| Appropriate gain | 539 (35.3) | 121 (31.7) | |||
| Excessive gain | 299 (19.6) | 62 (16.2) |
Bold values were statistically significant. b Fisher exact test.
AGA, appropriate for gestational age; BMI, body mass index; GWG, gestational weight gain; PSM, propensity score matching; SGA, small for gestational age.
Table 2.
Pregnancy characteristics of AGA group and SGA group matched according to 1:4 PSM analysis
| Variables | Items | AGA group (n=1528) | SGA group (n=382) | x2/t | P value |
| Parity | Nulliparous | 539 (35.3) | 195 (51) | 32.13 | <0.001 |
| Multiparous | 989 (64.7) | 187 (49) | |||
| Assisted reproductive technology | No | 1446 (94.6) | 362 (94.8) | 0.01 | 0.919 |
| Yes | 82 (5.4) | 20 (5.2) | |||
| Previous uterine scar | No | 1196 (78.3) | 337 (88.2) | 19.089 | <0.001 |
| Yes | 332 (21.7) | 45 (11.8) | |||
| Family history | No | 1367 (89.5) | 336 (88) | 2.809 | 0.422 |
| Hypertension | 76 (5) | 26 (6.8) | |||
| Diabetes | 46(3) | 13 (3.4) | |||
| Both | 39 (2.6) | 7 (1.8) | |||
| History of abortion or miscarriage | No | 896 (58.6) | 251 (65.7) | 6.393 | 0.041 |
| Spontaneous miscarriage | 348 (22.8) | 71 (18.6) | |||
| Induced abortions | 284 (18.6) | 60 (15.7) | |||
| History of preterm delivery | No | 1467 (96) | 368 (96.3) | 0.087 | 0.768 |
| Yes | 61 (4) | 14 (3.7) | |||
| History of macrosomia | No | 1481 (96.9) | 379 (99.2) | 6.29 | 0.012 |
| Yes | 47 (3.1) | 3 (0.8) | |||
| History of GDM | No | 1523 (99.7) | 382 (100) | / | 0.590b |
| Yes | 5 (0.3) | 0 (0) | |||
| History of fetal distress | No | 1512 (99) | 380 (99.5) | 0.897 | 0.343 |
| Yes | 16 (1) | 2 (0.5) | |||
| History of low birth weight | No | 1523 (99.7) | 376 (98.4) | / | 0.011b |
| Yes | 5 (0.3) | 6 (1.6) | |||
| Intrahepatic cholestasis of pregnancy | No | 1508 (98.7) | 377 (98.7) | 0 | 1 |
| Yes | 20 (1.3) | 5 (1.3) | |||
| Gestational hypertensive disorder | No | 1431 (93.7) | 324 (84.8) | 31.269 | <0.001 b |
| Gestational hypertension | 62 (4.1) | 31 (8.1) | |||
| Preeclampsia and eclampsia | 27 (1.8) | 22 (5.8) | |||
| Chronic hypertension with superimposed preeclampsia | 4 (0.3) | 3 (0.8) | |||
| Chronic hypertension (of any cause) | 4 (0.3) | 2 (0.5) | |||
| Hyperthyroid | No | 1487 (97.3) | 376 (98.4) | 1.576 | 0.209 |
| Yes | 41 (2.7) | 6 (1.6) | |||
| Hypothyroid | No | 1434 (93.8) | 349 (91.4) | 3.045 | 0.081 |
| Yes | 94 (6.2) | 33 (8.6) | |||
| Anaemia | No | 1149 (75.2) | 307 (80.4) | 4.508 | 0.034 |
| Yes | 379 (24.8) | 75 (19.6) | |||
| Polyhydramnios | No | 1517 (99.3) | 381 (99.7) | / | 0.479b |
| Yes | 11 (0.7) | 1 (0.3) | |||
| Oligohydramnios | No | 1490 (97.5) | 349 (91.4) | 32.314 | <0.001 |
| Yes | 38 (2.5) | 33 (8.6) | |||
| Ketonuria in first trimester (mmol/L) | <0.5 | 1049 (68.7) | 275 (72) | 9.963 | 0.007 |
| 0.5–3.9 | 336 (22) | 59 (15.4) | |||
| ≥4 | 143 (9.4) | 48 (12.6) | |||
| Ketonuria in second trimester (mmol/L) | <0.5 | 1090 (71.3) | 293 (76.7) | 4.903 | 0.086 |
| 0.5–3.9 | 308 (20.2) | 59 (15.4) | |||
| ≥4 | 130 (8.5) | 30 (7.9) | |||
| Elevated blood glucose in OGTT | One item | 482 (31.5) | 161 (42.1) | 24.605 | <0.001 |
| Two items | 878 (57.5) | 204 (53.4) | |||
| Three items | 168 (11.0) | 17 (4.5) | |||
| 75 g OGTT 0-hour glycaemia (mmol/L) | 4.83±0.48 | 4.64±0.44 | 7.187 | <0.001 | |
| 75 g OGTT 1-hour glycaemia (mmol/L) | 9.84±1.41 | 9.89±1.36 | −0.585 | 0.559 | |
| 75 g OGTT 2-hour glycaemia (mmol/L) | 8.06±1.59 | 7.83±1.58 | 2.586 | 0.01 | |
| FPG in the second trimester (mmol/L) | 4.87±0.56 | 4.73±0.49 | 4.372 | <0.001 | |
| 2-hour postprandial glucose in second trimester (mmol/L) | 6.09±1.30 | 5.7±1.14 | 5.825 | <0.001 | |
| HbA1c in the second trimester (mmol/L) | 5.26±0.36 | 5.28±0.35 | −1.008 | 0.314 | |
Bold values were statistically significant. b Fisher exact test.
AGA, appropriate weight for gestational age; FPG, fasting plasma glucose; HbA1c, haemoglobin A1c; OGTT, oral glucose tolerance test; the first trimester of pregnancy, 7–10 gestational weeks; the second trimester of pregnancy, 21–24 gestational weeks; the third trimester of pregnancy, 33–37 gestational weeks; PSM, propensity score matching; SGA, small for gestational age.
Multivariable logistic regression analysis for the factors of SGA
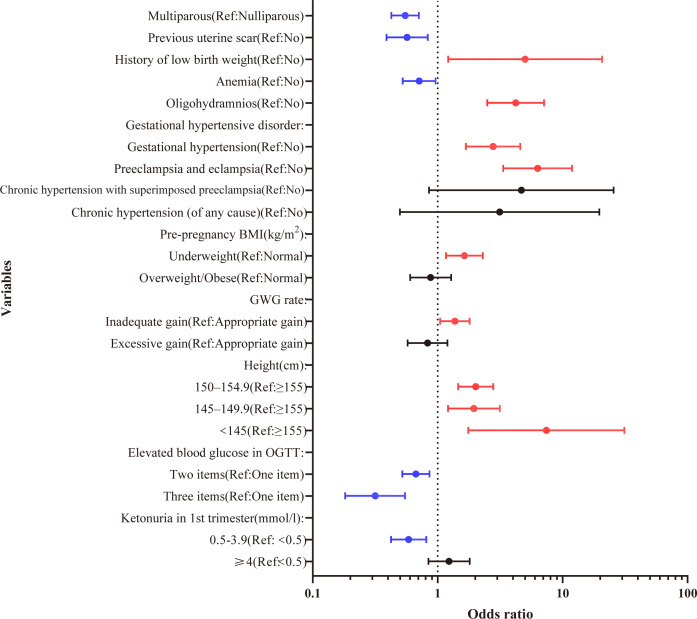
The multivariable analysis indicated that history of low birth weight (OR=5.01, 95% CI 1.21 to 20.72, p=0.026) was an independent risk factor for SGA. Mothers with gestational HD were more likely to have SGA (gestational hypertension: OR=2.78, 95% CI 1.68 to 4.59, p<0.001; preeclampsia and eclampsia: OR=6.31, 95% CI 3.35 to 11.91, p<0.001). The risk of SGA was fourfold greater in pregnant women with oligohydramnios than in women with normal amniotic fluid (OR=4.22, 95% CI 2.5 to 7.12, p<0.001). Mothers with lower height had a higher risk of SGA (150–154.9 cm: OR=2.02, 95% CI 1.46 to 2.79, p<0.001; 145–149.9 cm: OR=1.95, 95% CI 1.21 to 3.14, p=0.006; 145 cm: OR=7.42, 95% CI 1.76 to 31.25, p=0.006) compared with ≥155 cm height. Underweight pre-pregnancy had a 64% more chance of SGA (OR=1.64, 95% CI 1.17 to 2.3, p=0.004) than normal. Also, mothers who had inadequate weight gain during pregnancy had a 37% more chance of SGA than appropriate gain (OR=1.37, 95% CI 1.05 to 1.8, p=0.023).
However, the multivariate analysis also revealed that multiparous was a protective factor (OR=0.55, 95% CI 0.43 to 0.71, p<0.001) compared with nulliparity. The SGA risk was reduced by previous uterine scar experience (OR=0.57, 95% CI 0.39 to 0.83, p=0.004). Anaemia was associated with a decreased incidence of SGA (OR=0.71, 95% CI 0.53 to 0.96, p=0.027). Two or three items with elevated blood glucose values on OGTT showed a lower probability of SGA (OR=0.67, 95% CI 0.52 to 0.86, p=0.002; OR=0.32, 95% CI 0.18 to 0.55, p<0.001) than one elevated item. Ketonuria levels ranging from 0.5 to 3.9 mmol/L in the first trimester had a lower risk of SGA than <0.5 mmol/L (OR=0.59, 95% CI 0.42 to 0.81, p=0.001). The forest map of multivariate logistic regression analysis is shown in figure 2.
Figure 2.
Forest plot of the risk factors of small for gestational age (binary logistic regression analysis). BMI, body mass index; GWG, gestational weight gain; OGTT, oral glucose tolerance test.
Association between blood glucose level and the risk of SGA
We further explored the relationship between OGTT, glycaemic control level in the second trimester and SGA. Specifically, multivariate analysis adjusted for parity, previous uterine scar, history of low birth weight, history of abortion or miscarriage, history of macrosomia, gestational HD, oligohydramnios, anaemia, pre-pregnancy BMI, height, GWG rate and ketonuria in first trimester. In second trimester, 75 g OGTT 0 hour, 75 g OGTT 2 hours and 2-hour postprandial glucose were associated with a decreased risk for SGA (OR=0.4, 95% CI 0.29 to 0.55, p<0.001; OR=0.88, 95% CI 0.81 to 0.95, p=0.002; OR = 0.81, 95% CI 0.73 to 0.9, p<0.001). However, 75 g OGTT 0-hour glycaemia exhibited a stronger association with SGA outcomes than 2-hour OGTT and 2-hour postprandial glucose in the second trimester. In contrast, HbA1c in the second trimester was associated with an increased risk of SGA (OR=2.4, 95% CI 1.64 to 3.52, p<0.001) (table 3).
Table 3.
Logistic regression analysis for SGA based on maternal glycaemic parameters
| Variables | Crude | P value | Adjusted* | P value |
| OR (95% CI) | OR (95% CI) | |||
| 75 g OGTT 0-hour glycaemia | 0.39 (0.29 to 0.53) | <0.001 | 0.4 (0.29 to 0.55) | <0.001 |
| 75 g OGTT 1-hour glycaemia | 1.06 (0.97 to 1.15) | 0.217 | 1.04 (0.95 to 1.14) | 0.365 |
| 75 g OGTT 2-hour glycaemia | 0.88 (0.82 to 0.95) | 0.001 | 0.88 (0.81 to 0.95) | 0.002 |
| FPG in second trimester | 0.74 (0.57 to 0.97) | 0.026 | 0.77 (0.59 to 1.01) | 0.063 |
| 2-hour postprandial glucose in second trimester | 0.79 (0.71 to 0.88) | <0.001 | 0.81 (0.73 to 0.9) | <0.001 |
| HbA1c in the second trimester | 2.28 (1.6 to 3.25) | <0.001 | 2.4 (1.64 to 3.52) | <0.001 |
Bold values were statistically significant.
*Adjusted for parity, previous uterine scar, history of low birth weight, history of abortion or miscarriage, macrosomia, gestational hypertensive disorder, oligohydramnios, anaemia, pre-pregnancy body mass index, height, gestational weight gain rate and ketonuria in first trimester.
FPG, fasting plasma glucose; HbA1c, haemoglobin A1c; OGTT, oral glucose tolerance test; the third trimester of pregnancy, 33–37 gestational weeks.
Discussion
In this case–control study, several key maternal demographic characteristics (height, BMI and GWG rate), pregnancy characteristics (parity, previous uterine scar and history of LBW), pregnancy complications (HDs, oligohydramnios and anaemia), glycaemic laboratory parameters (ketonuria levels in the first trimester, 75 g OGTT 0 hour, 75 g OGTT 2 hours, 2-hour postprandial glucose and HbA1c in the second trimester) were identified as influencing factors for SGA in women with GDM.
Maternal height exerts the most significant influence. Our results confirmed that maternal stature below 145 cm is a strong indicator for SGA, aligning with previous studies.28 This may contribute to inadequate self-nutrition in GDM pregnant women who are of short stature. The transition to a sugar-controlled diet may have a significant impact on the adequate supply of nutrients for fetal growth. Further, both GWG and BMI serve as reflections of maternal nutritional status. Our study reveals that inadequate weight gain and underweight BMI were associated with an increased risk of SGA in women with GDM, consistent with prior research.29 This heightened risk may be attributed to pregnant women experiencing inadequate weight gain or being underweight, potentially indicating chronic malnutrition, which can be detrimental to fetal growth and development. Therefore, it is imperative that hospitals offer comprehensive health education, monitor pregnancy nutrition and implement personalised nutrition therapy for women diagnosed with GDM.
Nulliparous pregnant women with GDM exhibited an increased susceptibility to SGA births in our study, corroborating findings from a prior retrospective Chinese study.30 This heightened risk can be attributed to physiological disparities between nulliparous and multiparous women. Multiparous women showcased superior uteroplacental circulation, optimising oxygen and nutrient delivery to the fetus and creating a conducive environment for fetal growth.30 Conversely, nulliparous women displayed potential haemodynamic differences, including a higher pulsatility index of the uterine artery and elevated blood impedance, contributing to an elevated risk of SGA.31 32 Moreover, multiparous women were likely to possess a higher degree of maternal adaptation to gestational changes, encompassing improved blood volume expansion and hormonal regulation, thus fostering a favourable environment for fetal growth and diminishing the likelihood of SGA. Notably, differences in risk perception and prenatal care practices were apparent. Multiparous women, drawing on their experience, demonstrated proactive management skills for dietary changes and glycaemic control, resulting in more effective prenatal care and potentially reducing the risk of SGA. Conversely, nulliparous women’s relative inexperience might contribute to delayed or suboptimal prenatal care, impacting fetal growth outcomes.
Our research findings revealed an intriguing association, wherein a history of a previous uterine scar appeared to reduce the risk of SGA births among pregnant women with GDM. Remarkably, caesarean sections are widely preferred by Chinese women, with a national rate reaching 36.7% in 2018, the highest in Asia.33 In the context of Chinese obstetric practices, where multiparity is linked with a higher likelihood of opting for caesarean sections, it raises the possibility that the protective influence on SGA outcomes could be influenced by the prevalence of caesarean deliveries. It is important to emphasise that while a history of caesarean section may be associated with a lower risk of SGA, it does not imply that caesarean section itself is a recommended method for preventing SGA. The choice of delivery method should still be based on medical evaluations, taking into account the specific circumstances of the current pregnancy and medical indications.
Women with GDM face an increased risk for HD due to insulin resistance and the underlying pathology of the metabolic syndrome.34 HD is closely associated with birth weight,35 and when combined with GDM, it elevates the risk of adverse outcomes. This corresponds with our findings that gestational hypertension as well as preeclampsia and eclampsia are risk factors for delivering SGA in pregnant women with GDM. HD can induce spasms in maternal umbilical blood vessels and systemic small arteries, impacting maternal–fetal circulation and insufficient oxygen supply. Consequently, this affects the intrauterine growth and development of the fetus.36 The presence of HDs, characterized by a decrease in serum vascular endothelial growth factor and placental growth factor levels, alongside an increase in soluble fms-like tyrosine kinase-1 levels, may reflect underlying placental dysfunction and are related to inhibition in fetal growth and development.37 38 Oligohydramnios, often seen in conjunction with HDs,39 may indicate complicated pregnancies, signifying chronic suboptimal placental function.40 Such conditions could reduce fetal resources and are associated with SGA. Thus, maternal blood pressure should be closely monitored and regular ultrasound examinations should be performed to assess changes in pregnancy status.
Contrary to earlier research, this study discovered that maternal anaemia during pregnancy reduces the incidence of SGA.41 One possible explanation is that women with GDM are particularly attentive to their diet, incorporating supplementation recommended by their obstetricians to address anaemia. Consequently, they may effectively mitigate the risk of SGA through appropriate nutritional support. Besides, the effect of anaemia on pregnancy outcomes varies between gestational periods. Therefore, further research is needed to investigate the effect of haemoglobin concentration on SGA at different gestational ages.
Maternal glycaemic parameters significantly influence fetal growth, as highlighted by findings from the HAPO study. Pregnant women with elevated glucose levels face a higher risk of adverse pregnancy outcomes. This association is driven by various mechanisms. First, heightened glucose levels can stimulate increased fetal insulin production, promoting excessive fetal growth and contributing to macrosomia.17 Conversely, elevated glucose levels may, in some instances, impair placental function, leading to reduced nutrient and oxygen supply to the fetus, ultimately resulting in growth restriction and the birth of SGA infants.42 Our study found that GDM women with two or three elevated glucose values, as opposed to just one, may experience a decreased risk of SGA. Besides, higher OGTT 0 hour and OGTT 2 hours were found to be significant predictors of SGA when the glucose values were analysed as continuous variables. This may contribute to within the mild elevation range of blood glucose levels; blood glucose passes through the placental circulation to the fetus and extra glucose in the fetus is stored as body fat.43 There may be a protective mechanism ensuring that the fetus receives adequate nutrients within the normal range. However, this does not imply that higher blood glucose levels are better. When blood glucose rises to a certain extent, adaptive responses may be triggered, leading to the occurrence of SGA. Therefore, GDM women with elevated OGTT 0-hour and OGTT 2-hour levels are less likely to deliver SGA infants. However, they should be aware of more severe disturbance in glucose metabolism and insulin sensitivity and the potential for delivering high birth weight newborns. In addition, GDM women with low OGTT 0 hour and OGTT 2 hours do not require excessively strict glucose control throughout pregnancy, but should be concerned about the occurrence of FGR. Therefore, personalised monitoring is crucial for assessing maternal blood glucose levels, allowing for the adjustment of diet, exercise and insulin management strategies based on their glycaemic status.
Our study identified an association between delivering SGA in pregnant women with GDM and 2-hour postprandial glucose in the second trimester. Measuring 2-hour postprandial glucose helps evaluate the effectiveness of dietary modifications and glycaemic control strategies.44 In clinical practice, pregnant women are advised to control their glycaemic levels through dietary adjustments when diagnosed with GDM. However, due to fear of insulin and lack of knowledge about GDM treatment options, some women may follow an overly strict diet. Consequently, maternal glucose regulation is inadequate, which can lead to fetal undergrowth.21Hence, pregnant women diagnosed with GDM should be warned of the potential risk of SGA if they are found to have low glucose values. Besides, compared with the late pregnancy period, timely blood glucose testing in the second trimester provides a longer time window. More attention should be paid to glucose status during this period. Understanding the glycaemic status is a crucial step in adjusting the diet and exercise plan to achieve stable blood glucose levels, ensuring normal fetal development, and avoiding SGA.
The multifactorial analysis revealed the association between elevated HbA1c levels in the second trimester and an increased risk of SGA, suggesting a potential impact of long-term glucose control on fetal outcomes. However, this finding differs from a previous study45 and contradicts the results of instantaneous glycaemic measures (OGTT and 2-hour postprandial glucose) in our study. This discrepancy may be attributed to the curvilinear relationship between HbA1c and fetal weight. Specifically, normal fetal weight may occur at low HbA1c levels, while moderately raised levels may result in macrosomia, and very high HbA1c levels may be associated with severe intrauterine growth restriction.46 Future research could explore the relationship between glycaemic control and birth weight using unrestricted cubic splines or subgroup analyses to evaluate their correlation. This approach would contribute to a more comprehensive understanding of the intricate relationship between maternal glycaemic and fetal outcomes.
Limitation
There are a few limitations to our analysis. First, data regarding women’s history of smoking and drinking was not recorded. Although the incidence of smoking and drinking among pregnant women is low due to Chinese customs, smoking and drinking experience may be potential contributors to SGA. Second, data was collected from a single hospital and may not be representative of other areas. Third, this study is a case–control study even though a PSM analysis was conducted to minimise the bias. Lastly, this study lies in the inability to accurately differentiate FGR from overall SGA during the grouping process, aligning with the specific objectives of the study. Future research endeavours could consider employing more specific diagnostic criteria and focusing explicitly on FGR, offering a more comprehensive understanding of these distinct fetal growth conditions.
Conclusion
SGA infants born to women with GDM are the result of a multifactorial interaction, including maternal demographic characteristics, pregnancy characteristics, pregnancy complications, and glycaemic laboratory parameters. Notably, SGA was correlated with glycaemic control levels. It is difficult to reverse once SGA has occurred; perinatal monitoring and antenatal care are crucial for identifying risk factors that can help predict and prevent SGA.
Supplementary Material
Acknowledgments
Thanks to all participants for their valuable contribution to this study.
Footnotes
Contributors: JL and YP: writing—original draft preparation, writing—reviewing and editing, visualisation; XJ: supervision and conceptualisation. QZ and XC: methodology and validation. YZ, RL and LH: investigation and data curation. XJ is responsible for the overall content as the guarantor. All authors have approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: The study was supported by the Nursing Research Fund of Fujian Maternity and Child Health Hospital (YCXH 22-13).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: JL and YP contributed equally. JL and YP are joint first authors.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data used to support the findings of this study are available from the corresponding author upon request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Ethics Committee (No. 2019-161). Given all maternal and neonatal data were extracted from the hospital electronic medical record system by a unique identifier with no participant involved in the design, the written informed consent was waived.
References
- 1.Reitzle L, Schmidt C, Heidemann C, et al. Gestational diabetes in Germany: development of screening participation and prevalence [Robert Koch-Institut, report]. J Health Monit 2021;6:3–18. 10.25646/8325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomedicine & Pharmacotherapy 2021;143:112183. 10.1016/j.biopha.2021.112183 [DOI] [PubMed] [Google Scholar]
- 3.Gao C, Sun X, Lu L, et al. Prevalence of gestational diabetes mellitus in Mainland China: A systematic review and meta-analysis. J Diabetes Investig 2019;10:154–62. 10.1111/jdi.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol 1954;16:330–42. 10.1530/acta.0.0160330 [DOI] [PubMed] [Google Scholar]
- 5.Langmia IM, Kräker K, Weiss SE, et al. Cardiovascular programming during and after diabetic pregnancy: role of Placental dysfunction and IUGR. Front Endocrinol 2019;10:215. 10.3389/fendo.2019.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayak AU, Vijay AMA, Indusekhar R, et al. Association of Hypoglycaemia in screening oral glucose tolerance test in pregnancy with low birth weight fetus. World J Diabetes 2019;10:304–10. 10.4239/wjd.v10.i5.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Xiao H, Yang Y, et al. Demographic and clinical features of small-for-gestational-age infants born to mothers with gestational diabetes mellitus. Front Pediatr 2021;9:741793. 10.3389/fped.2021.741793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilliod RA, Cheng YW, Snowden JM, et al. The risk of Intrauterine fetal death in the small-for-gestational-age fetus. Am J Obstet Gynecol 2012;207:318. 10.1016/j.ajog.2012.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee ACC, Katz J, Blencowe H, et al. National and regional estimates of term and Preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 2013;1:e26–36. 10.1016/S2214-109X(13)70006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anne RP, Vardhelli V, Oleti TP, et al. Propensity-matched comparison of very Preterm Small- and appropriate-for-gestational-age neonates. Indian J Pediatr 2022;89:59–66. 10.1007/s12098-021-03878-3 [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Chen H, Xi F, et al. Association between maternal blood lipids levels during pregnancy and risk of small-for-gestational-age infants. Sci Rep 2020;10:19865. 10.1038/s41598-020-76845-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuste Gómez A, Ramos Álvarez M del P, Bartha JL. Influence of diet and lifestyle on the development of gestational diabetes mellitus and on perinatal results. Nutrients 2022;14:2954. 10.3390/nu14142954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barquiel B, Herranz L, Martínez-Sánchez N, et al. Increased risk of neonatal complications or death among neonates born small for gestational age to mothers with gestational diabetes. Diabetes Res Clin Pract 2020;159:107971. 10.1016/j.diabres.2019.107971 [DOI] [PubMed] [Google Scholar]
- 14.Esakoff TF, Guillet A, Caughey AB. Does small for gestational age worsen outcomes in gestational diabetics. The Journal of Maternal-Fetal & Neonatal Medicine 2017;30:890–3. 10.1080/14767058.2016.1193142 [DOI] [PubMed] [Google Scholar]
- 15.Neimark E, Wainstock T, Sheiner E, et al. Long-term cardiovascular hospitalizations of small for gestational age (SGA) offspring born to women with and without gestational diabetes mellitus (GDM Gynecol Endocrinol 2019;35:518–24. 10.1080/09513590.2018.1541233 [DOI] [PubMed] [Google Scholar]
- 16.Alves LNR, Pereira M, Dos Santos JA, et al. Investigation of maternal Polymorphisms in genes related to glucose homeostasis and the influence on birth weight: a cohort study. J Pediatr (Rio J) 2022;98:296–302. 10.1016/j.jped.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 18.Cao X, Wang Z, Yang C, et al. Comprehensive intensive therapy for Chinese gestational diabetes benefits both newborns and mothers. Diabetes Technol Ther 2012;14:1002–7. 10.1089/dia.2012.0142 [DOI] [PubMed] [Google Scholar]
- 19.Morampudi S, Balasubramanian G, Gowda A, et al. The challenges and recommendations for gestational diabetes mellitus care in India: A review. Front Endocrinol (Lausanne) 2017;8:56. 10.3389/fendo.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dassios T, Greenough A, Leontiadi S, et al. Admissions for Hypoglycaemia after 35 weeks of gestation: perinatal predictors of cost of stay. J Matern Fetal Neonatal Med 2019;32:448–54. 10.1080/14767058.2017.1381905 [DOI] [PubMed] [Google Scholar]
- 21.Leng J, Hay J, Liu G, et al. Small-for-gestational age and its association with maternal blood glucose, body mass index and stature: a perinatal cohort study among Chinese women. BMJ Open 2016;6:e010984. 10.1136/bmjopen-2015-010984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delibas IB, Tanriverdi S, Cakmak B. Does reactive Hypoglycemia during the 100 G oral glucose tolerance test adversely affect perinatal outcomes Ginekol Pol 2018;89:25–9. 10.5603/GP.a2018.0005 [DOI] [PubMed] [Google Scholar]
- 23.Shinohara S, Uchida Y, Hirai M, et al. Relationship between maternal Hypoglycaemia and small-for-gestational-age infants according to maternal weight status: a retrospective cohort study in two hospitals. BMJ Open 2016;6:e013749. 10.1136/bmjopen-2016-013749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu W, Hu H, Zhao W, et al. Current status of Antenatal care of pregnant Women-8 provinces in China, 2018. BMC Public Health 2021;21:1135. 10.1186/s12889-021-11154-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzger BE, Gabbe SG, Persson B. International Association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai L, Deng C, Li Y, et al. Population-based birth weight reference Percentiles for Chinese twins. Ann Med 2017;49:470–8. 10.1080/07853890.2017.1294258 [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Cao Z, Zhang Y, et al. Birthweight Percentiles for twin birth neonates by gestational age in China. Sci Rep 2016;6:31290. 10.1038/srep31290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanam R, Lee AC, Mitra DK, et al. Maternal short stature and under-weight status are independent risk factors for Preterm birth and small for gestational age in rural Bangladesh. Eur J Clin Nutr 2019;73:733–42. 10.1038/s41430-018-0237-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Q-X, Wang H-W, Jiang X-M, et al. Prepregnancy body mass index and gestational weight gain are associated with maternal and infant adverse outcomes in Chinese women with gestational diabetes. Sci Rep 2022;12:2749. 10.1038/s41598-022-06733-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin L, Lu C, Chen W, et al. Parity and the risks of adverse birth outcomes: a retrospective study among Chinese. BMC Pregnancy Childbirth 2021;21:257. 10.1186/s12884-021-03718-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prefumo F, Bhide A, Sairam S, et al. Effect of parity on second-trimester uterine artery Doppler flow velocity and Waveforms. Ultrasound Obstet Gynecol 2004;23:46–9. 10.1002/uog.908 [DOI] [PubMed] [Google Scholar]
- 32.Derwig I, Lythgoe DJ, Barker GJ, et al. Association of Placental perfusion, as assessed by magnetic resonance imaging and uterine artery Doppler ultrasound, and its relationship to pregnancy outcome. Placenta 2013;34:885–91. 10.1016/j.placenta.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 33.Qiao J, Wang Y, Li X, et al. A lancet Commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet 2021;397:2497–536. 10.1016/S0140-6736(20)32708-2 [DOI] [PubMed] [Google Scholar]
- 34.Baumfeld Y, Novack L, Wiznitzer A, et al. Pre-conception Dyslipidemia is associated with development of Preeclampsia and gestational diabetes mellitus. PLoS One 2015;10:e0142462. 10.1371/journal.pone.0142462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, Li Z, Ye R, et al. Preconception blood pressure and risk of low birth weight and small for gestational age: A large cohort study in China. Hypertension 2016;68:873–9. 10.1161/HYPERTENSIONAHA.116.07838 [DOI] [PubMed] [Google Scholar]
- 36.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013;382:273–83. 10.1016/S0140-6736(13)60311-6 [DOI] [PubMed] [Google Scholar]
- 37.Tang Y, Ye W, Liu X, et al. VEGF and sFLT-1 in serum of PIH patients and effects on the Foetus. Exp Ther Med 2019;17:2123–8. 10.3892/etm.2019.7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badagionis M, Sergentanis TN, Pervanidou P, et al. Preeclampsia and cerebral palsy in offspring. Children (Basel) 2022;9:385. 10.3390/children9030385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabie N, Magann E, Steelman S, et al. Oligohydramnios in complicated and uncomplicated pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49:442–9. 10.1002/uog.15929 [DOI] [PubMed] [Google Scholar]
- 40.Dastjerdi MV, Ghahghaei-Nezamabadi A, Tehranian A, et al. The effect of Sildenafil on pregnancy outcomes in pregnant women with idiopathic borderline Oligohydramnios: A randomized controlled trial. JFRH 2022;16:124–31. 10.18502/jfrh.v16i2.9482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu D, Li S, Zhang B, et al. Maternal hemoglobin concentrations and birth weight, low birth weight (LBW and small for gestational age (SGA): findings from a prospective study in Northwest China. Nutrients 2022;14:858. 10.3390/nu14040858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fasoulakis Z, Koutras A, Antsaklis P, et al. Intrauterine growth restriction due to gestational diabetes: from pathophysiology to diagnosis and management. Medicina (Kaunas) 2023;59:1139. 10.3390/medicina59061139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntyre HD, Fuglsang J, Kampmann U, et al. Hyperglycemia in pregnancy and women’s health in the 21st century. Int J Environ Res Public Health 2022;19:16827. 10.3390/ijerph192416827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monnier L, Colette C. Target for Glycemic control. Diabetes Care 2009;32(suppl_2):S199–204. 10.2337/dc09-S310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Y, Zhang X. Association between maternal glucose/lipid metabolism parameters and abnormal newborn birth weight in gestational diabetes complicated by Preeclampsia: A retrospective analysis of 248 cases. Diabetes Ther 2020;11:905–14. 10.1007/s13300-020-00792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rackham O, Paize F, Weindling AM. Cause of death in infants of women with Pregestational diabetes mellitus and the relationship with Glycemic control. Postgrad Med 2009;121:26–32. 10.3810/pgm.2009.07.2026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078325supp001.pdf (124.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data used to support the findings of this study are available from the corresponding author upon request.