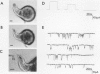
Abstract
We used an ultraviolet laser to rupture a small region of cell wall of a polarized Fucus spiralis rhizoid cell and gained localized access to the plasma membrane at the growing apex. Careful control of cell turgor enabled a small portion of plasma membrane-bound cytoplasm to be exposed. Gigaohm seals allowing single-channel recordings were obtained with a high success rate using this method with conventional patch clamp techniques.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elzenga J. T., Keller C. P., Van Volkenburgh E. Patch clamping protoplasts from vascular plants : method for the quick isolation of protoplasts having a high success rate of gigaseal formation. Plant Physiol. 1991 Dec;97(4):1573–1575. doi: 10.1104/pp.97.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley-Grenot K., Assmann S. M. Evidence for G-Protein Regulation of Inward K+ Channel Current in Guard Cells of Fava Bean. Plant Cell. 1991 Sep;3(9):1037–1044. doi: 10.1105/tpc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley K., Laver D., Walker N. A. Whole-cell and single-channel currents across the plasmalemma of corn shoot suspension cells. J Membr Biol. 1991 Apr;121(1):11–22. doi: 10.1007/BF01870647. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harold F. M. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol Rev. 1990 Dec;54(4):381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf D. L., Kloareg B., Quatrano R. S. Cell wall is required for fixation of the embryonic axis in Fucus zygotes. Science. 1988 Jan 8;239(4836):187–190. doi: 10.1126/science.3336780. [DOI] [PubMed] [Google Scholar]
- Lew R. R., Serlin B. S., Schauf C. L., Stockton M. E. Red light regulates calcium-activated potassium channels in mougeotia plasma membrane. Plant Physiol. 1990 Mar;92(3):822–830. doi: 10.1104/pp.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N., Ehrenstein G., Iwasa K., Bare C., Mischke C. Ion channels in plasmalemma of wheat protoplasts. Science. 1984 Nov 16;226(4676):835–838. doi: 10.1126/science.6093255. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Wilson K. J. Properties of Single K and Cl Channels in Asclepias tuberosa Protoplasts. Plant Physiol. 1987 Oct;85(2):413–418. doi: 10.1104/pp.85.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenseel M. H., Jaffe L. F. Return to normal of Fucus egg membrane after microelectrode impalement. Exp Cell Res. 1974 Nov;89(1):55–62. doi: 10.1016/0014-4827(74)90186-4. [DOI] [PubMed] [Google Scholar]