Abstract
Introduction
Batoclimab, a fully human monoclonal antibody that inhibits the neonatal fragment crystallisable receptor, has shown promising phase 2 clinical trial results in patients with generalised myasthenia gravis (gMG).
Methods and analysis
In this phase 3, randomised, quadruple-blind, placebo-controlled study, adults with gMG will be randomised 1:1:1 to induction therapy with batoclimab 680 mg, batoclimab 340 mg, or placebo, administered once weekly (QW) for 12 weeks as a subcutaneous injection. The primary endpoint is the change from baseline to week 12 on the Myasthenia Gravis Activities of Daily Living (MG-ADL) score. Batoclimab-treated patients achieving a ≥2-point improvement from baseline on MG-ADL at week 10 or week 12 will be re-randomised to maintenance treatment with batoclimab 340 mg QW, batoclimab 340 mg every other week (Q2W), or placebo for 12 weeks; batoclimab-treated patients with a <2-point improvement at week 10 and week 12 will be switched to placebo for the maintenance period and discontinued thereafter. Placebo-treated patients from the induction period will be re-randomised to batoclimab 340 mg QW or Q2W in the maintenance period. All patients who complete the maintenance period and achieve a ≥2-point improvement from baseline in MG-ADL during ≥1 of the final 2 visits of the induction and/or maintenance periods will continue their current batoclimab dose (or switch to batoclimab 340 mg QW for those on placebo) for a 52-week long-term extension (LTE-1). Patients who complete LTE-1 may enter a second, optional 52-week LTE (LTE-2).
Ethics and dissemination
This trial is being conducted in accordance with the International Council for Harmonisation Guideline for Good Clinical Practice, the Declaration of Helsinki, and each site’s Institutional Review Board/Independent Ethics Committee. All patients must provide written informed consent. Results from this study will be published in peer-reviewed journals and presented at national and global conferences.
Trial registration number
Keywords: IMMUNOLOGY, MYASTHENIA, RANDOMISED TRIALS, FC RECEPTOR
WHAT IS ALREADY KNOWN ON THIS TOPIC
Neonatal fragment crystallisable receptor (FcRn) inhibitors are an emerging class of treatments for myasthenia gravis (MG) that work by blocking FcRn-mediated immunoglobulin G (IgG) recycling, resulting in clearance of IgG autoantibodies. This represents a more targeted therapeutic approach compared with the broad impact on the immune system of conventional MG treatments, potentially offering patients a safer and more effective alternative for the long-term management of a disease characterised by fluctuating symptoms. Batoclimab is a fully human anti-FcRn monoclonal antibody being developed as a low-volume subcutaneous injection for the treatment of several IgG-mediated autoimmune diseases, with promising phase 2a trial results in patients with MG.
WHAT THIS STUDY ADDS
The phase 3 FLEX trial of batoclimab in generalised MG (gMG) was designed to meet specific patient needs during different phases of the disease, including rapid reduction of symptoms during flares, prevention of myasthenic exacerbation/crisis, and long-term maintenance of remission using the lowest effective dose.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The FLEX study is expected to provide pivotal data supporting batoclimab as a treatment option in patients with gMG, as well as valuable practical insights around the implementation of an induction-maintenance regimen with batoclimab.
Introduction
Myasthenia gravis (MG) is a rare autoimmune disease targeting the neuromuscular junction, leading to fatigable muscle weakness that can manifest as ptosis, diplopia, dysarthria, dysphonia, dysphagia, extremity weakness and dyspnoea due to respiratory muscle weakness.1 The patient burden is significant and may impact nearly every aspect of life, including physical functioning, emotional well-being, relationships, family planning and ability to work.2 In patients with acetylcholine receptor antibody (AChRAb)-positive disease, conventional therapeutic strategies primarily include symptomatic treatment with acetylcholinesterase inhibitors, along with corticosteroids and steroid-sparing immunosuppressive agents, complement inhibitors and thymectomy.3–5 Plasma exchange (PLEX) and intravenous immunoglobulin are used primarily to treat myasthenic worsening or exacerbation/crisis.3 5 In patients with muscle-specific kinase antibody (MuSKAb)-positive disease, corticosteroids and other immunotherapies (ie, PLEX, rituximab) are the mainstay treatments but there remains a paucity of available proven therapies.4 5
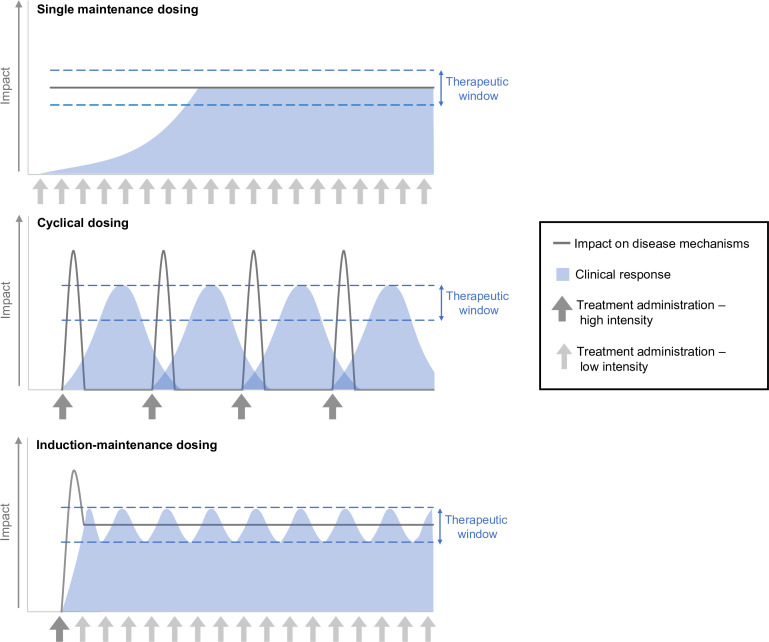
The ideal treatment strategy for MG would generate a rapid clinical response that can be maintained over time, while balancing the possibility for side effects as well as the cost and potential inconvenience of parenteral therapy. This may be best achieved with an induction-maintenance approach. In contrast to single-maintenance or cyclical approaches in which patients consistently receive the same dose with each administration, an induction-maintenance strategy comprises a high-potency therapy to rapidly reduce disease manifestations, followed by another, typically less-intensive regimen in which the lowest effective dose is used to maintain the remission of symptoms and disease control (figure 1). First used in oncology,6 7 this strategy has been successfully applied to other disease states. In patients with clinically active rheumatoid arthritis, for example, an induction-maintenance approach comprising tumour necrosis factor inhibitor treatment plus methotrexate was associated with reductions in disease activity and increased rates of remission compared with methotrexate treatment alone.8 In patients with relapsing-remitting multiple sclerosis, induction-maintenance therapy with rituximab9 was associated with improvements from baseline in disability score and annualised relapse rate. In patients with refractory MG, there is evidence that induction therapy with rituximab can reduce the dose of corticosteroids and/or immunosuppressant therapy needed to maintain minimal manifestation status.10
Figure 1.
Potential approaches to treatment administration in MG. MG, myasthenia gravis.
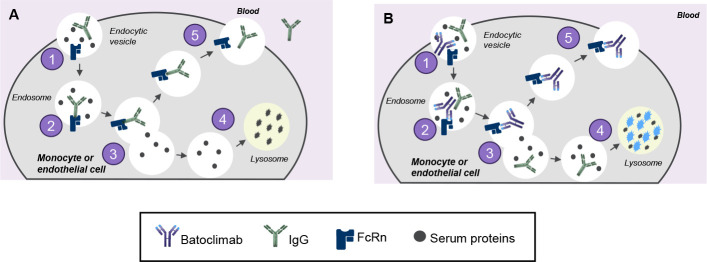
Neonatal fragment crystallisable receptor (FcRn) inhibitors are an emerging class of treatments for MG that work by binding to FcRn, thereby blocking FcRn-mediated immunoglobulin G (IgG) recycling and leading to lysosomal degradation and clearance of IgG autoantibodies (figure 2).11 12 This mechanism of action offers a more targeted therapeutic approach compared with the broad impact on the immune system of conventional treatments. Currently, there are two FcRn inhibitors approved for treatment in MG, including the human IgG1-derived Fc fragment, efgartigimod13 and the recombinant humanised IgG4P monoclonal antibody, rozanolixizumab.14
Figure 2.
MOA of batoclimab. (A) FcRn maintains levels of IgG in circulation by preventing IgG degradation. (1) IgG enters cells via fluid-phase pinocytosis; (2) FcRn binds antibodies in a pH-dependent manner with high binding affinity at mild acidic conditions (pH 6.0) present in the endosome; (3) FcRn-IgG complexes are sorted from unbound proteins; (4) unbound proteins are trafficked to the lysosome for degradation; (5) IgG is recycled back to the cell surface and released into circulation. (B) Selective depletion and lysosomal degradation of pathogenic IgG antibodies via FcRn inhibition with batoclimab. (1) IgG and batoclimab are taken up into cells via endocytosis. (2) Batoclimab preferentially binds to FcRn in endosomes (batoclimab has high binding affinity at high and low pH). (3) FcRn-batoclimab complexes are sorted from unbound proteins including unbound IgG. (4) Non-receptor bound IgG are degraded in the lysosomes. (5) Batoclimab may remain bound to FcRn, further inhibiting IgG recycling. FcRn, neonatal fragment crystallisable receptor; IgG, immunoglobulin G; MOA, mechanism of action.
Batoclimab is a fully human anti-FcRn monoclonal antibody that is being developed as a low-volume subcutaneous (SC) injection for the treatment of a variety of IgG-mediated autoimmune disorders, including MG,15 thyroid eye disease,16–18 chronic inflammatory demyelinating polyneuropathy19 and Graves’ disease.20 21 In MG, phase 2 trials conducted in North America22 and China23 showed that batoclimab was associated with significant reductions in serum IgG and AChRAb levels. Clinical outcomes, including Myasthenia Gravis Activities of Daily Living (MG-ADL), Quantitative Myasthenia Gravis (QMG) and Myasthenia Gravis Composite scores also favoured batoclimab. Phase 2 safety outcomes showed that batoclimab was generally well tolerated; treatment-emergent adverse events (TEAEs) were mild to moderate in severity and most commonly included injection-site reactions, headache, dizziness, hypercholesterolaemia, hypernatraemia, urinary tract infection and peripheral oedema.22 23
The phase 3 FLEX trial, which is currently recruiting patients, was designed to better understand the clinical impact of batoclimab in patients with generalised MG (gMG), including the effectiveness of an induction-maintenance approach to management of disease. The objectives of this article are to describe the design of the FLEX trial and to articulate the rationale for various design elements.
Methods and analysis
Overall design
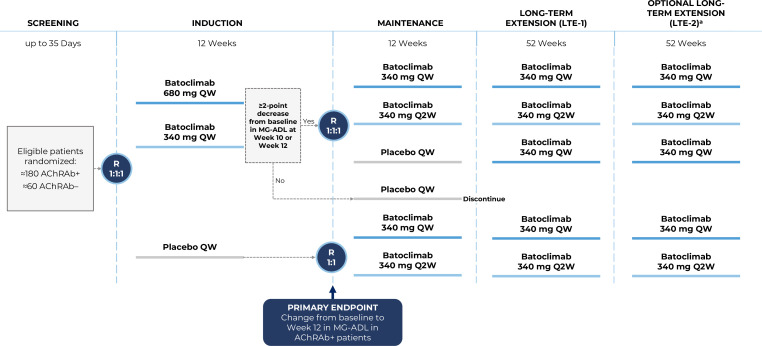
The FLEX trial (NCT05403541) is a phase 3, pivotal, multinational, randomised, quadruple-blind, placebo-controlled study to assess the efficacy and safety of batoclimab in adult patients with gMG. This study includes five periods: (1) a 35-day screening period; (2) a 12-week placebo-controlled induction period; (3) a 12-week maintenance period; (4) a 52-week long-term extension period (LTE-1) and (5) an additional 52-week optional long-term extension (LTE-2) (figure 3). The total study duration will be approximately 133 weeks.
Figure 3.
Phase 3 FLEX study design of batoclimab in patients with gMG. aWhere required by local regulations, participants may continue to receive batoclimab beyond LTE-2. These participants may continue in the study until marketing authorisation in that specific region, the development programme is discontinued, or batoclimab becomes available through another patient programme (whichever occurs first). AChRAb, acetylcholine receptor antibody; gMG, generalised myasthenia gravis; MG-ADL, Myasthenia Gravis Activities of Daily Living; QW, weekly; Q2W, every other week; R, randomisation.
Eligibility criteria
Key eligibility criteria include age≥18 years; diagnosis of mild to severe gMG (defined as an Myasthenia Gravis Foundation of America classification of II, III, or IVa) at screening; and confirmation of acetylcholine receptor antibodies (AChRAb+), muscle-specific kinase antibodies (MuSKAb+) or low-density lipoprotein receptor-related protein 4 antibodies (LRP4Ab+) at screening. Participants who are seronegative to known autoantibodies will be eligible if they have a history of abnormal neuromuscular transmission demonstrated by single-fibre electromyography or repetitive nerve stimulation, and either a positive edrophonium chloride test or a demonstrated improvement in gMG signs with oral cholinesterase inhibitors. In addition, patients will be required to have a QMG score≥11 and an MG-ADL score≥5 at both the screening and baseline visits, with no more than 50% of QMG and MG-ADL scores attributed to ocular findings/symptoms. All patients must currently be receiving treatment or have a history of treatment with corticosteroids, cholinesterase inhibitors, or non-steroidal immunosuppressants.
Patients who have experienced a myasthenic crisis within 3 months before screening, had a thymectomy within 6 months before screening or have a planned thymectomy during the study period, or who have an active or untreated malignant thymoma will be ineligible for participation. In addition, patients with any of the following laboratory abnormalities at screening will be excluded: total IgG<6 g/L; platelet count<100 000/μL; albumin level<3.0 g/dL; aspartate aminotransferase or alanine aminotransferase levels≥2.5× the upper limit of normal (ULN); bilirubin≥1.5 × ULN; absolute neutrophil count<1000 cells/mm3; fasting low-density lipoprotein cholesterol (LDL)≥190 mg/dL in patients with no history of cardiovascular disease or ≥160 mg/dL in patients with a history of coronary, cerebral, or peripheral artery disease; and stage 3B chronic kidney disease. Finally, any patient with New York Heart Association Class 3 or 4 heart failure, or who has had a cardiovascular event or revascularisation procedure within 6 months before screening, will be excluded.
Sample size and power calculations
Sample size and power calculations were performed based on assumptions of efficacy derived from the phase 2a trial of batoclimab in MG22 and the phase 3 ADAPT trial of efgartigimod,24 as well as an expected dropout rate of 5%. Based on this, a sample size of 180 AChRAb+ patients (60 per group) and up to 60 AChRAb− patients (20 per group) was determined to have sufficient power to detect treatment differences across the primary and secondary endpoints (table 1).
Table 1.
Assumptions and power calculations for primary and secondary endpoints
| Endpoints | Assumptions | Effective sample size/Group | Power |
| Change from baseline to week 12 in MG-ADL score for AChRAb+ patients |
|
≈60 | >90% |
| Change from baseline to week 12 in QMG for AChRAb+ patients |
|
≈60 | >90% |
| Change from weeks 12 to 24 in MG-ADL for AChRAb+ randomised withdrawal patients |
|
≈30 | 80%–<90% |
| Proportion of AChRAb+ patients with a ≥3-point improvement from baseline to week 12 in QMG |
|
≈60 | >90% |
| Proportion of AChRAb+ patients with an MG-ADL score of 0 or 1 by week 12 |
|
≈60 | >90% |
| Change from baseline to week 12 in MG-ADL score for AChRAb– patients |
|
Batoclimab: ≈40 Placebo: ≈20 |
70%–<80% |
Note: expected dropout rate is 5%.
AChRAb, acetylcholine receptor antibodies; MG-ADL, Myasthenia Gravis Activities of Daily Living; QMG, quantitative myasthenia gravis.
Randomisation and patient flow
Induction period
Eligible patients from ≈110 investigative sites worldwide will be randomised 1:1:1 in the induction period to batoclimab 680 mg once weekly (QW), batoclimab 340 mg QW, or placebo QW using an interactive response system. Randomisation will be stratified according to baseline QMG score (<16 or ≥16), current non-steroidal immunosuppressant use (yes/no), and serological status (AChRAb+ or AChRAb−).
Maintenance period
At the completion of the induction period, batoclimab-treated patients who achieve a ≥2-point improvement from baseline in MG-ADL score at weeks 10 or 12 will be re-randomised 1:1:1 to batoclimab 340 mg QW, batoclimab 340 mg every other week (Q2W), or placebo QW in the maintenance period, comprising the randomised withdrawal population. Randomisation for this group will be stratified according to induction period treatment assignment and change from baseline in QMG score at week 12 (<5 or ≥5 points). Allowing patients to enter the maintenance period based on their response at week 10 or week 12 of induction provides them with the best chance to continue active treatment, which is an important consideration given the fluctuating nature of MG symptoms. Patients initially assigned to batoclimab who have a <2-point improvement from baseline in MG-ADL at week 10 and week 12 will be switched to blinded placebo treatment during the maintenance period, which is necessary to maintain the integrity of the induction period study blind. Importantly, these patients will have access to rescue medication during this time and will be discontinued from the study at the end of the maintenance period. Patients assigned to placebo during the induction period will be randomised 1:1 to batoclimab 340 mg QW or Q2W, with randomisation stratified by QMG score at the start of the maintenance period (<16 or ≥16), current non-steroidal immunosuppressant use (yes/no), and serological status (AChRAb+ or AChRAb−).
Long-term extensions 1 and 2
Patients completing the maintenance period will be eligible to enter LTE-1 only if they experienced a ≥2-point improvement from baseline in MG-ADL during ≥1 of the last 2 visits during either the induction or the maintenance period. Patients meeting these criteria who received batoclimab 340 mg QW or Q2W during the maintenance period will continue on the same dose for the extension, while those who received placebo will be switched to batoclimab 340 mg QW.
All patients completing LTE-1 will be eligible to continue their current treatment in LTE-2, which will continue to examine the long-term efficacy and safety of batoclimab. Where required by local regulations, participants may continue to receive batoclimab beyond LTE-2 by repeating the LTE-2 dosing and assessment schedule. These participants may continue in the study until marketing authorisation in that specific region, the development programme is discontinued, or batoclimab becomes available through another patient programme (whichever occurs first).
Blinding
This study will employ a quadruple blind (sponsor, investigator, independent assessor and participant). Study treatment will be supplied in prefilled syringes containing batoclimab 340 mg or placebo in 2 mL of solution. In order to maintain the blind, all patients will receive two SC injections of study drug and/or placebo (depending on treatment assignment) every week during the induction period and one SC injection every week during the maintenance and extension periods. The role of the blinded investigator at each site will be to assess the patient’s general condition, review available laboratory data, query for potential adverse events (AEs), and conduct a physical examination not including the efficacy endpoints. The blinded assessor will be responsible solely for assessing efficacy outcomes and will not have access to any other information regarding the patient’s history or general condition. Discussions between the investigator and the assessor will be limited to actionable results of scoring. Of note, batoclimab treatment is associated with alterations in the serum levels of several analytes, including albumin, total cholesterol, LDL, alkaline phosphatase and IgG.23 25 Since knowledge of these values could compromise the study blind, they will not be shared with investigators unless required to protect patient safety. Instead, an unblinded independent data safety monitoring committee will review safety data regularly throughout the study, and notify investigators to important changes in laboratory parameters based on predefined trigger alerts outlined in the study protocol.
Concomitant therapy and rescue medication use
During the induction and maintenance periods, patients should continue on their entry dose of corticosteroids. For those receiving <15 mg of prednisone daily or equivalent (including<30 mg every other day [EOD]), daily excursions in either direction will be limited to ≤2.5 mg (≤5 mg for EOD regimen), and for participants receiving 15–30 mg of prednisone daily or equivalent (including≥30 mg to≤60 mg EOD), excursions are limited to ≤5 mg (≤10 mg for EOD regimen). However, the dose may be adjusted down for safety reasons. During LTE-1, upward excursions from the entry dose of corticosteroids will be limited to ≤5 mg of daily prednisone or equivalent (≤10 mg for EOD regimens) from weeks 24–52; after week 52, upward excursions will be limited to ≤10 mg of daily prednisone (≤10 mg for EOD regimens). There will be no limits on downward excursions of steroid use during LTE-1. During LTE-2, there will be no limits on upward or downward excursions.
In addition to ongoing corticosteroid treatment, patients will also be allowed to continue therapy with cholinesterase inhibitors and immunosuppressants (ie, azathioprine, mycophenolate mofetil, methotrexate, cyclosporine, cyclophosphamide, tacrolimus) that they were receiving at the time of study entry, as outlined in the eligibility criteria. However, any changes to the dose or frequency of these medications, or introduction of new standard-of-care medications during the induction or maintenance periods will not be allowed. In addition, use of the following medications will be prohibited: any Ig treatment (given via SC, IV, or intramuscular route); PLEX; other investigational products, FcRn inhibitors, or monoclonal antibodies (besides those used to treat COVID-19); or live vaccines (non-live/seasonal COVID-19 vaccinations and/or emergency vaccinations [eg, tetanus] will be allowed). No new B-cell inhibitors, complement inhibitors, or non-steroidal immunosuppressants may be started once a patient enters the study.
During all study periods, the need for rescue medication will be assessed in all patients experiencing an exacerbation of gMG (defined as a ≥4-point increase from baseline in QMG score for ≥2 consecutive visits or a ≥6-point increase between 2 consecutive visits). Patients requiring an excluded medication as a rescue treatment to manage MG signs and symptoms will be discontinued from study treatment. Patients discontinuing study drug because of rescue medication use during the induction or maintenance periods will be asked to complete all remaining visits for that study period, and then will be discontinued from the study following completion of the early termination/follow-up visit. During LTE-1 and LTE-2, investigators may elect to treat patients requiring rescue with open-label batoclimab (680 mg QW for 4 weeks, followed by 340 mg QW) in lieu of the above treatment options. These patients would have their blinded study medication stopped but continue in the study. Patients who, in the opinion of the investigator, are not responding to open-label batoclimab, or those who receive rescue treatment with an excluded medication during LTE-1 or LTE-2, will be discontinued from the study following completion of the early termination/follow-up visit.
Outcomes
The primary objective of the FLEX study is to evaluate the efficacy of batoclimab 680 mg QW and 340 mg QW versus placebo as induction therapy in AChRAb+ patients, based on change from baseline to Week 12 in MG-ADL score. Change in MG-ADL from baseline to week 12 in AChRAb– patients will be included as a secondary endpoint. Additional secondary endpoints during the induction period include change from baseline to week 12 in QMG score in AChRAb+ patients, and the proportions of AChRAb+ patients who achieve a ≥3-point improvement from baseline in QMG and an MG-ADL score of 0 or 1 (minimal symptom expression) at week 12.
The impact of batoclimab 340 mg QW and Q2W versus placebo as a maintenance therapy will be examined in AChRAb+ randomised withdrawal patients. Change from week 12 to week 24 in MG-ADL will be examined as a secondary endpoint. Change in QMG from week 12 to week 24, the proportion of patients achieving a ≥3-point improvement in QMG from induction period baseline to week 24, and the proportion of patients achieving a ≥2-point improvement in MG-ADL from induction period baseline to week 24 will be included as tertiary endpoints.
Long-term outcomes with batoclimab 340 mg QW and Q2W will be examined using exploratory endpoints in AChRAb+ patients. These include changes in QMG and MG-ADL from week 24 to various timepoints through week 76, the proportion of patients with a ≥3-point improvement in QMG from induction period baseline to timepoints between week 24 and week 76, and the proportion of patients with a ≥2-point improvement in MG-ADL from induction period baseline to timepoints between week 24 and week 76.
Safety outcomes include AEs, including the proportions of patients with TEAEs and laboratory-related TEAEs. Five categories of AEs of special interest have been identified in potential relation to batoclimab treatment, including infection, oedema, hypoalbuminaemia, hypercholesterolaemia and hypogammaglobulinaemia. Severity of AEs will be graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 and assessed for a causal relationship to the study drug by the study investigator. Serious AEs (SAEs) will also be recorded, with SAEs defined as those events considered by the investigator or the sponsor to be life-threatening; result in persistent or significant disability/incapacity or substantial disruption of the ability to conduct normal life functions; result in congenital abnormality or birth defect; require hospitalisation or prolongation of hospitalisation; result in death; or lead to other medically important events. Changes from baseline in vital signs and laboratory values will also be recorded.
Statistical analysis
All analyses will be conducted using Statistical Analysis Software V.9.4 or greater. The analysis of efficacy endpoints in the induction period will be primarily based on full analysis set (FAS)-1, comprising all randomised participants who receive any study treatment in the induction period. Efficacy assessments during the maintenance period will be primarily based on FAS-2, defined as all re-randomised participants who receive any study treatment during the maintenance period. The primary efficacy endpoint will be analysed using a mixed model for repeated measures approach (MMRM), with change from baseline in MG-ADL at each timepoint being the outcome and baseline MG-ADL score, nonsteroidal immunosuppressant use (yes/no), baseline QMG score (<16 or ≥16), visit, treatment group, and visit-by–treatment group interaction as covariates. Secondary and tertiary continuous efficacy endpoints will be analysed using an MMRM similar to that described for the primary efficacy analysis; categorical endpoints will be analysed using the Cochran-Mantel-Haenszel method with adjustment for stratification factors in the induction (non-steroidal immunosuppressant use [yes/no], baseline QMG [<16 or ≥16]) or maintenance periods (induction period treatment assignment [340 or 680 mg], improvement in QMG from baseline to week 12 [<5 points or ≥5 points]), as appropriate.
For patients who discontinue study drug and do not receive a prohibited rescue medication, analysis of efficacy outcomes post-treatment discontinuation will proceed based on observed values. For patients who discontinue study drug and are treated with a prohibited rescue medication, the worst available blinded score post-treatment discontinuation will be assigned for visits occurring through the end of the study period. Of note, the worst available score may be obtained at an unscheduled visit prior to the administration of rescue medication or occur after the use of rescue medication in patients who continue to deteriorate. At intercurrent visits that are post-treatment discontinuation but prior to use of rescue medication, the actual observed value at each visit will be used.
Safety outcomes will be assessed by study period, based on all patients who receive treatment in the induction (SAF1), maintenance (SAF2), LTE-1 (SAF3), and LTE-2 (SAF4) periods. AEs, electrocardiograms, vital signs and safety laboratory data will be reviewed and summarised on an ongoing basis during the trial to evaluate the safety of participants.
Discussion
The phase 3 FLEX trial is expected to provide pivotal evidence supporting the use of batoclimab in patients with gMG. Earlier findings from phase 2 trials showed that SC batoclimab 680 mg and 340 mg were generally well tolerated and associated with positive pharmacodynamic and clinical outcomes.22 23 Moreover, recent topline results from a phase 3 study in China showed that patients who received batoclimab were more likely to achieve sustained improvements on MG-ADL than those who received placebo.26 27 The FLEX trial will expand on these findings, using a larger patient population and longer duration of time than previous trials, and incorporating an induction-maintenance strategy.
FLEX was designed using a patient-centred approach, with each phase specifically tailored to address patient needs. Patients entering the study have active disease, and the induction period will investigate the optimal dose of batoclimab required to achieve rapid clinical responses, including on measures of physical functioning, disease severity, activities of daily living, mental health and quality of life. While analyses primarily focus on the AChRAb+ population, our study is also expected to include a small number of MuSKAb+ patients (≈20 total); primary and secondary endpoints will be examined specifically in these patients as part of a subgroup analysis.
Patients will be initially randomised 1:1:1 to batoclimab 680 mg QW, batoclimab 340 mg QW, or placebo QW, ensuring that 2/3 patients receive active treatment from the start. Patients initially assigned to placebo will be re-randomised to active treatment in the maintenance period, ensuring that every patient who enrols in the study has an opportunity to receive active treatment. The doses of batoclimab for the induction period were chosen based on phase 2 data in MG and thyroid eye disease, which demonstrated reductions in IgG as early as 1 week after the first dose, as well as promising clinical outcomes.22 25 Both doses have been associated with reductions in serum albumin, which is expected based on the MOA of batoclimab, and elevations in lipids, including LDL-C, which led to the early termination of the phase 2b study in thyroid eye disease. However, these changes were well tolerated in most patients, reversible on discontinuation and associated more strongly with the 680 mg dose. Importantly, the FLEX trial employs batoclimab 680 mg as a temporary induction regimen, with lower doses used in the maintenance and follow-up periods, to balance rapid efficacy with long-term safety.
The maintenance period of FLEX will examine the viability of two potential maintenance doses of batoclimab (340 mg QW or Q2W) in patients who respond to induction treatment. Owing to the fluctuating nature of MG symptoms, patients will be re-randomised to the maintenance period if they achieved a ≥2-point improvement from baseline in MG-ADL at week 10 or week 12 of the induction period, giving each participant the best chance to enter the maintenance phase. Importantly, these patients will be re-randomised prior to entering the maintenance period. Whereas larger trials may opt for a parallel group design in which treatment switches are predefined at the time of randomisation, in smaller studies, there may be non-study-drug-related events that can impact the balance of patient characteristics across groups after they complete induction. Re-randomisation of patients prior to entering the maintenance phase, including stratification for previous dose assignment, provides a rebaselining of the populations that ensures greater parity across groups.
In addition to investigating the efficacy of different maintenance doses of batoclimab, results from the randomised withdrawal population are also expected to provide insight on the durability of the induction effect in the absence of adequate maintenance treatment. Phase 3 evidence from another FcRn inhibitor, efgartigimod, suggests a short hysteresis loop between IgG suppression and clinical response, with an estimated lag time of approximately 1 week.24 Assuming a similar effect will occur with batoclimab (although this has yet to be confirmed), patients in the randomised withdrawal population are expected to lose any clinical benefits gained during the induction period by the end of the maintenance period if on placebo or an inadequate maintenance dose. These findings would support a chronic dosing regimen as opposed to cyclic or intermittent dosing.
Patients who do not respond to induction treatment with batoclimab 680 or 340 mg QW will receive blinded placebo during the maintenance period. These individuals are considered very unlikely to experience any benefit from maintenance treatment with batoclimab at the risk of continued drug-induced alterations in several laboratory analytes. Assignment to placebo is also necessary to maintain the induction period blinding. Of note, patients participating in phase 3 clinical trials of other FcRn inhibitors could have received blinded treatment with placebo for 24–26 weeks,24 28 which is similar to the duration of time a patient could potentially receive inadequate treatment in the FLEX trial (ie, placebo or an insufficient dose of batoclimab). Critically, these patients, along with all other study participants, will have access to rescue medication throughout the trial in the event of an MG exacerbation. Further examination of this group may reveal useful insights about the characteristics of patients unlikely to respond to treatment.
The objective of LTE-1 and LTE-2 is to optimise disease control. In many patients, it is expected that this will be achieved with maintenance treatment. However, even with ongoing therapy, patients may experience changes in their symptoms. The possibility of adjusting the dose over time according to disease activity is therefore an attractive option. In the FLEX study, patients experiencing a flare in LTE-1 or LTE-2 will be permitted to use open-label batoclimab 680 mg QW for 4 weeks as a rescue, followed by maintenance treatment with batoclimab 340 mg QW. This feature may help guide physicians with respect to optimising control in patients whose disease waxes and wanes.
In summary, the phase 3 FLEX study is expected to provide practical guidance around the application of an induction-maintenance strategy in patients with gMG. FcRn inhibitors are still a new class of drugs at the present time, and their use in the real world is currently informed by existing trial designs and outcomes from those trials. In MG, patients and their healthcare providers are frequently confronted with two distinct and important clinical scenarios: (1) signs and symptoms are poorly controlled or worsening, warranting a change in treatment; or (2) signs and symptoms are well controlled, and a reduction in the intensity of treatment is necessary to decrease or reduce the risk of untoward side effects and burden of treatment, including cost. Trials of FcRn inhibitors that employ cyclical24 29 or single maintenance30 dosing regimens are not designed to adequately address these needs. The main drawback of cyclical dosing is that the decision for additional treatment after the initial cycle is based on disease worsening in patients, while single-maintenance dosing trials do not offer the possibility to adjust the dose in response to changes in disease activity. Unfortunately, this leaves the community with a major knowledge gap. In contrast, the induction-maintenance strategy used in FLEX is designed to provide rapid and continuous efficacy that can be safely maintained over time using the lowest effective dose, with the option for reinduction or adjustment of the maintenance dose in response to individual patient circumstances.
Ethics and dissemination
This trial is being conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice; the Declaration of Helsinki; all applicable national, state and local laws and regulations; and each site’s Institutional Review Board/Independent Ethics Committee. All patients will be required to provide written informed consent. The results from this study will be submitted for publication in high-impact medical journals and presentation at national and international congresses, regardless of outcome.
Plain language summary
MG is a rare disease with unpredictable weakness. Symptoms may affect the eyes, face, throat, limbs and/or breathing muscles. An ideal medicine for MG would be able to quickly improve symptoms and keep them under control in the future, without causing unnecessary risks. This article describes a study of batoclimab, a potential medicine for MG. The study will include four parts. In the first part, patients will receive one of two possible doses of batoclimab (680 mg or 340 mg) or placebo. These treatments will be injected under the skin once per week for 12 weeks. The goal of treatment during the first part of the study is to improve symptoms. In the second part of the study, patients will receive batoclimab 340 mg, either once a week or once every 2 weeks, or placebo for 12 weeks. During the second part of the study, researchers will look at whether patients retain improvements in their symptoms from the first part. In the third and fourth parts of the study, which are each 52 weeks long, patients will keep taking the same dose of batoclimab from the second part (or switch to batoclimab 340 mg once per week if taking placebo during the second part) to see whether they maintain symptom control in the long term. Patients who have a flare in symptoms during the third or fourth parts of the study will be able to receive batoclimab 680 mg once per week for 4 weeks, followed by maintenance treatment with batoclimab 340 mg once per week. This provides the opportunity to maximise symptom control. The results of this study will provide important information on whether batoclimab helps control symptoms of MG. It will also help doctors and their patients better understand how to more optimally manage a disease that fluctuates over time.
Acknowledgments
The authors would like to thank Liz Rockstein, PhD, Jennifer Scwhinn, RPh, and Paola Mina-Osorio, MD, PhD, employees of Immunovant, for their support in the organisation and coordination of the manuscript. We also thank all patients, their families and caregivers for participating in this study.
Footnotes
Contributors: All authors: substantial contribution to the design of the study, conceptualisation of the manuscript, critical revisions of the draft manuscript, approved final draft.
Competing interests: MB: reports grants from the National Institutes of Health, the Muscular Dystrophy Association, the ALS Association, Alexion and Immunovant; as well as consulting fees for Alector, Alexion, Annexon, Arrowhead, Biogen, Cartesian, Denali, Eli Lilly, Horizon, Immunovant, Janssen, NMD Pharma, Novartis, Orphazyme A/S, Roche, Sanofi, Takeda, UCB and UniQure. The University of Miami has licensed intellectual property to Biogen to support design of the ATLAS study. HW: receives honoraria for acting as a member of Scientific Advisory Boards for Abbvie, Alexion, argenx, Bristol Myers Squibb/Celgene, Janssen, Merck, Novartis, and Sandoz; and receives speaker honoraria and travel support from Alexion, Biogen, Bristol Myers Squibb, Genzyme, Merck, Neurodiem, Novartis, Ology, Roche, TEVA, and WebMD Global. Professor Wiendl also acts as a paid consultant for Abbvie, Actelion, argenx, BD, Biogen, Bristol Myers Squibb, EMD Serono, Fondazione Cariplo, Gossamer Bio, Idorsia, Immunic, Immunovant, Janssen, Lundbeck, Merck, NexGen, Novartis, PSI CRO, Roche, Sanofi, The Swiss Multiple Sclerosis Society, UCB, and Worldwide Clinical Trials. His research is funded by the Deutsche Forschungsgesellschaft (DFG), Deutsche Myasthenie Gesellschaft e.V., European Union, Alexion, Amicus Therapeutics, argenx, Biogen, CSL Behring, F. Hoffmann - La Roche, Genzyme, Merck KgaA, Novartis, Roche Pharma, UCB Biopharma. RJN: reports research support from the National Institutes of Health, Genentech, Alexion Pharmaceuticals, argenx, Annexon Biosciences, Ra Pharmaceuticals (now UCB S.A.), the Myasthenia Gravis Foundation of America, Momenta Pharmaceuticals (now Janssen), Immunovant, Grifols, S.A., and Viela Bio (Horizon Therapeutics plc). Dr. Nowak has also served as a consultant and advisor for Alexion Pharmaceuticals, argenx, Cabaletta Bio, Cour Pharmaceuticals, Ra Pharmaceuticals (now UCB S.A.), Immunovant, Momenta Pharmaceuticals (now Janssen), and Viela Bio (Horizon Therapeutics plc). YZ: Employee of Immunovant. WM: Employee of Immunovant.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Institutional Review Board/Independent Ethics committee approval is required from each individual study site before the study is initiated, and this is stated in the manuscript. As this study is currently recruiting, we do not have the specific names of the IRBs at this time. Participants gave informed consent to participate in the study before taking part.
References
- 1. Hehir MK, Silvestri NJ. Generalized myasthenia gravis classification, clinical presentation, natural history, and epidemiology. Neurol Clin 2018;36:253–60. 10.1016/j.ncl.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 2. Lehnerer S, Jacobi J, Schilling R, et al. Burden of disease in myasthenia gravis: taking the patient’s perspective. J Neurol 2022;269:5688–9. 10.1007/s00415-022-11290-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farmakidis C, Pasnoor M, Dimachkie MM, et al. Treatment of myasthenia gravis. Neurol Clin 2018;36:311–37. 10.1016/j.ncl.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology 2021;96:114–22. 10.1212/WNL.0000000000011124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology 2016;87:419–25. 10.1212/WNL.0000000000002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Von Essen CF, Joseph LBM, Simon GT, et al. Sequential chemotherapy and radiation therapy of Buccal mucosa carcinoma in South India: methods and preliminary results. American Journal of Roentgenology 1968;102:530–40. 10.2214/ajr.102.3.530 [DOI] [PubMed] [Google Scholar]
- 7. Stephens FO. CRAB” care and cancer chemotherapy. Med J Aust 1976;2:41–4, 10.5694/j.1326-5377.1976.tb117617.x [DOI] [PubMed] [Google Scholar]
- 8. Emamikia S, Arkema EV, Györi N, et al. Induction maintenance with tumour necrosis factor-inhibitor combination therapy with discontinuation versus methotrexate monotherapy in early rheumatoid arthritis: a systematic review and meta-analysis of efficacy in randomised controlled trials. RMD Open 2016;2:e000323. 10.1136/rmdopen-2016-000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao D, Zhao C, Lu J, et al. Efficacy and safety of repeated low-dose Rituximab therapy in relapsing-remitting multiple sclerosis: A retrospective case series study. Multiple Sclerosis and Related Disorders 2023;70:104518. 10.1016/j.msard.2023.104518 [DOI] [PubMed] [Google Scholar]
- 10. Singh N, Goyal V. Rituximab as induction therapy in refractory myasthenia gravis: 18-month follow-up study. J Neurol 2019;266:1596–600. 10.1007/s00415-019-09296-y [DOI] [PubMed] [Google Scholar]
- 11. Nelke C, Spatola M, Schroeter CB, et al. Neonatal FC receptor–targeted therapies in neurology. Neurotherapeutics 2022;19:729–40. 10.1007/s13311-021-01175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peter H-H, Ochs HD, Cunningham-Rundles C, et al. Targeting Fcrn for Immunomodulation: benefits, risks, and practical considerations. J Allergy Clin Immunol 2020;146:479–91. 10.1016/j.jaci.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. VYVGART® (efgartigimod alfa-fcab) injection for intravenous use . Prescribing information. Argenx BV. 2022. Available: https://www.argenx.com/product/vyvgart-prescribing-information.pdf [Accessed 24 Oct 2023].
- 14. RYSTIGGO® (rozanolixizumab-noli) injection for subcutaneous use . Prescribing information. UCB, Inc; 2023. Available: https://www.ucb-usa.com/RYSTIGGO-prescribing-information.pdf [Accessed 24 Oct 2023]. [Google Scholar]
- 15. Immunovant Sciences . A study of RVT-1401 in myasthenia gravis (MG) patients. Clinicaltrials.Gov Identifier: Nct03863080. 2021. Available: https://clinicaltrials.gov/ct2/show/NCT03863080 [Accessed 24 Oct 2023].
- 16. Immunovant Sciences . Extension study to assess Batoclimab in participants with thyroid eye disease. Clinicaltrials.Gov Identifier: Nct05517447. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT05517447 [Accessed 24 Oct 2023].
- 17. Immunovant Sciences . Study to assess Batoclimab in participants with active thyroid eye disease. Clinicaltrials.Gov Identifier: Nct05524571. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT05524571 [Accessed 24 Oct 2023].
- 18. Immunovant Sciences . Study to assess Batoclimab in participants with active thyroid eye disease (B). Clinicaltrials.Gov Identifier: Nct05517421. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT05517421 [Accessed 24 Oct 2023].
- 19. Immunovant Sciences GmbH . To assess efficacy and safety of Batoclimab in adult participants with active CIDP. Nct05581199. n.d. Available: https://clinicaltrials.gov/ct2/show/NCT05581199
- 20. Immunovant Sciences . Study of RVT-1401 for the treatment of patients with moderate to severe active graves. Available: https://clinicaltrials.gov/ct2/show/NCT03922321 [Accessed 24 Oct 2023].
- 21. Immunovant Sciences . ASCEND GO-2: study of RVT-1401 for the treatment of participants with active, moderate to severe graves’ Ophthalmopathy (GO). Clinicaltrials.Gov Identifier: Nct03938545. 2022. Available: https://clinicaltrials.gov/ct2/show/NCT03938545 [Accessed 24 Oct 2023].
- 22. Nowak RJ, Breiner A, Bril V, et al. Subcutaneous batoclimab in generalized myasthenia gravis: results from a phase 2a trial with an open-label extension. Ann Clin Transl Neurol 2023;Dec 7. 10.1002/acn3.51946 [Epub ahead of print Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan C, Duan R-S, Yang H, et al. Therapeutic effects of Batoclimab in Chinese patients with generalized myasthenia gravis: A double-blinded, randomized, placebo-controlled phase II study. Neurol Ther 2022;11:815–34. 10.1007/s40120-022-00345-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howard JF, Bril V, Vu T, et al. Safety, efficacy, and tolerability of Efgartigimod in patients with generalised myasthenia gravis (ADAPT): a Multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2021;20:526–36. 10.1016/S1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 25. Kahaly GJ, Dolman PJ, Wolf J, et al. Proof-of-concept and randomized, placebo-controlled trials of an Fcrn inhibitor, Batoclimab, for thyroid eye disease. J Clin Endocrinol Metab 2023;108:3122–34. 10.1210/clinem/dgad381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harbour Biomed ANNOUNCES positive Topline results from phase III trial of Batoclimab for treatment of generalized myasthenia gravis. 2023. Available: https://www.harbourbiomed.com/news/207.html [Accessed 24 Oct 2023].
- 27. Harbour BioMed (Guangzhou) Co., Ltd . Evaluate the efficacy and safety of Hbm9161(Hl161) subcutaneous injection in patients with generalized MG patients (MG). Clinicaltrials.Gov Identifier: Nct05039190. 2023. Available: https://clinicaltrials.gov/study/NCT05039190 [Accessed 24 Oct 2023].
- 28. Ramchandren S, Sanga P, Burcklen M, et al. Vivacity MG phase 3 study: clinical trial of Nipocalimab administered to adults with generalized myasthenia gravis. Neurology 2022;99(23_Supplement_2):S41. 10.1212/01.wnl.0000903328.46907.49 [DOI] [Google Scholar]
- 29. UCB Biopharma SRL . A study to evaluate Rozanolixizumab in study participants with generalized myasthenia gravis. Clinicaltrials.Gov Identifier: Nct04650854. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT04650854
- 30. A study of Nipocalimab administered to adults with generalized myasthenia gravis. Clinicaltrials.Gov Identifier: Nct04951622. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT04951622 [Accessed 24 Oct 2023].