Abstract
Question
Tricyclic antidepressants are used to treat depression worldwide, but the adverse effects have not been systematically assessed. Our objective was to assess the beneficial and harmful effects of all tricyclic antidepressants for adults with major depressive disorder.
Study selection and analysis
We conducted a systematic review with meta-analysis and trial sequential analysis. We searched CENTRAL, MEDLINE, Embase, LILACS and other sources from inception to January 2023 for randomised clinical trials comparing tricyclic antidepressants versus placebo or ‘active placebo’ for adults with major depressive disorder. The primary outcomes were depressive symptoms measured on the 17-item Hamilton Depression Rating Scale (HDRS-17), serious adverse events and quality of life. The minimal important difference was defined as three points on the HDRS-17.
Findings
We included 103 trials randomising 10 590 participants. All results were at high risk of bias, and the certainty of the evidence was very low or low. All trials only assessed outcomes at the end of the treatment period at a maximum of 12 weeks after randomisation. Meta-analysis and trial sequential analysis showed evidence of a beneficial effect of tricyclic antidepressants compared with placebo (mean difference −3.77 HDRS-17 points; 95% CI −5.91 to −1.63; 17 trials). Meta-analysis showed evidence of a harmful effect of tricyclic antidepressants compared with placebo on serious adverse events (OR 2.78; 95% CI 2.18 to 3.55; 35 trials), but the required information size was not reached. Only 2 out of 103 trials reported on quality of life and t-tests showed no evidence of a difference.
Conclusions
The long-term effects of tricyclic antidepressants and the effects on quality of life are unknown. Short-term results suggest that tricyclic antidepressants may reduce depressive symptoms while also increasing the risks of serious adverse events, but these results were based on low and very low certainty evidence.
PROSPERO registration number
CRD42021226161.
Keywords: psychiatry, adult psychiatry, depression & mood disorders
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Tricyclic antidepressants are used to treat major depressive disorder worldwide.
The National Institute for Health and Care Excellence and the Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders recommend tricyclic antidepressants for patients with chronic or melancholic depression or as an alternative for patients who do not benefit from newer antidepressants.
Previous reviews have not systematically assessed all adverse effects for all tricyclic antidepressants, so it remains unclear whether the potential benefits outweigh the harmful effects of tricyclic antidepressants.
WHAT THIS STUDY ADDS
The long-term effects of tricyclic antidepressants and the effects on quality of life and suicides or suicide attempts are unknown.
Short-term results suggest that tricyclic antidepressants may reduce depressive symptoms, while also increasing the risks of serious adverse events, but these results are based on low and very low certainty evidence.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
It is a cause for concern that there are no data from randomised clinical trials on the long-term effects of tricyclic antidepressants and only low and very low certainty evidence on short-term effects given that so many people use these drugs for several years.
Background
Major depressive disorder is a psychiatric condition characterised by depressed mood and diminished interest or pleasure.1 Major depressive disorder is estimated to affect more than 264 million people globally2 and is associated with a high risk of suicidal behaviour.3–5 Tricyclic antidepressants are used in the treatment of major depressive disorder worldwide.6–12 Although selective serotonin reuptake inhibitors are generally recommended as first-line treatment for major depressive disorder, the National Institute for Health and Care Excellence (NICE) and the Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders recommend tricyclic antidepressants for patients with chronic or melancholic depression or as an alternative for patients who do not benefit from newer antidepressants.13 14 The WHO Model List of Essential Medicines also includes the tricyclic antidepressant amitriptyline as one of just two essential antidepressants for the treatment of depressive disorders.15
It has previously been shown that antidepressants reduce depressive symptoms with statistically significant effects, but it is uncertain how important these effects are to patients and whether they represent genuine pharmacological effects or just amplified placebo effects.16–18 One systematic review suggests that amitriptyline has larger effects than other antidepressants compared with placebo.19 However, previous reviews have not systematically assessed suicides, suicide attempts and all serious and non-serious adverse events for all tricyclic antidepressants,19–23 so it remains unclear whether the harmful effects of tricyclic antidepressants outweigh the potential beneficial effects.
Objective
Our objective was to assess the beneficial and harmful effects of all tricyclic antidepressants versus placebo or ‘active placebo’ in the treatment of adults with major depressive disorder.
Study selection and analysis
We report this systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (online supplemental file 1).24 25 The methodology used in this systematic review is described in detail in The Cochrane Handbook of Systematic Reviews of Interventions and our protocol,26 27 which was registered in the PROSPERO database prior to the systematic literature search (ID: CRD42021226161).
bmjment-2023-300730supp001.pdf (4.9MB, pdf)
Search strategy and selection criteria
Electronic searches
An experienced information specialist searched the Cochrane Central Register of Controlled Trials (CENTRAL), Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (Embase), Latin American and Caribbean Health Sciences Literature (LILACS), PsycINFO, Science Citation Index Expanded (SCI-EXPANDED), Social Sciences Citation Index (SSCI), Chinese Biomedical Literature Database (CBM), China Network Knowledge Information (CNKI), Chinese Science Journal Database (VIP), Wafang Database, Conference Proceedings Citation Index—Science (CPCI-S) and Conference Proceedings Citation Index—Social Science and Humanities (CPCI-SSH) to identify relevant trials. We searched all databases from their inception to 27 January 2023. For a detailed search strategy for all electronic databases, see online supplemental file 2.
Searching other resources
To identify unpublished trials, we also searched clinical trial registers, websites of pharmaceutical companies and websites of US Food and Drug Administration (FDA) and European Medicines Agency (EMA). We requested FDA, EMA and national medicines agencies to provide all publicly releasable information about relevant trials of antidepressants submitted for marketing approval, including clinical study reports. Additionally, we hand-searched conference abstracts from psychiatry conferences.
Selection criteria
We included randomised clinical trials irrespective of language, publication status, publication year and publication type. Participants had to be adults with a primary diagnosis of major depressive disorder as defined by standardised diagnostic criteria, such as Diagnostic and Statistical Manual of Mental Disorders1 or International Classification of Diseases.28 As experimental intervention, we included any tricyclic antidepressant. As control intervention, we included placebo, ‘active placebo’ or no intervention.
Data extraction and risk of bias assessment
Two authors (CBK and PF) independently screened relevant trials. Seven authors working in pairs (CBK, PF, JJP, ATK, SJ, FS and MB) independently extracted data using a standardised data extraction sheet and assessed risk of bias based on the Cochrane Risk of Bias tool, V.2 (RoB 2).29 30 Discrepancies were resolved through internal discussion or, if required, through discussion with a third author (JCJ).
Outcomes and subgroup analyses
The primary outcomes were depressive symptoms measured on the 17-item Hamilton Depression Rating Scale (HDRS-17), serious adverse events (as defined by the International Conference on Harmonisation—Good Clinical Practice (ICH-GCP) guidelines: any untoward medical occurrence that resulted in death, was life-threatening, required hospitalisation or prolonging of existing hospitalisation and resulted in persistent or significant disability or jeopardised the participant)31 and quality of life. Secondary outcomes were the proportion of participants with either suicides or one or more suicide attempts and non-serious adverse events. Exploratory outcomes were suicidal ideation, depressive symptoms measured on the Montgomery-Asberg Depression Rating Scale (MADRS),32 the Beck’s Depression Inventory (BDI)33 or HDRS-6,34 35 treatment response (defined as a 50% reduction from baseline) and remission (as defined by trialists). Outcomes were assessed at the end of treatment and at maximum follow-up. We also planned several subgroup analyses.27
When extracting adverse events, we assumed the events were non-serious unless otherwise specified by the trialists. If the trialists did not report the proportion of non-serious adverse events, we used the most common non-serious adverse event for this proportion to potentially avoid double-counting participants with more than one type of non-serious adverse events. When serious adverse events were not reported according to the ICH-GCP definition (ie, if the events were not defined as ‘serious adverse events’ or if the definition of serious adverse events was unclear),31 we categorised any adverse event clearly fulfilling the ICH-GCP definition as a serious adverse event. The assessment was made by two review authors who received the full list of all events and discussed the severity of each event. The authors were blinded and therefore did not know whether the events were recorded in an experimental or placebo group. If the authors disagreed on the severity of a specific event, they would discuss this with a third author. We used the same systematic approach in all trials, reflecting standard procedures that have been employed in multiple previous reviews.36–47 If trialists did not report an overall proportion of serious adverse events according to the ICH-GCP definition,31 we used the most common serious adverse event for this proportion to potentially avoid double-counting participants with more than one type of serious adverse events.
Assessment of statistical and clinical significance
We performed meta-analyses according to the Cochrane Handbook for Systematic Reviews of Interventions,29 Keus et al,48 and the eight-step procedure by Jakobsen et al.49 We planned to assess a total of five main outcomes, and therefore considered a p value of 0.016 or less as the threshold for statistical significance.49 We assessed the intervention effects with both random-effects (Hartung-Knapp-Sidik-Jonkman)50 and fixed-effect model meta-analyses (Mantel-Haenszel for dichotomous outcomes and inverse variance for continuous outcomes).29 51 We primarily reported the most conservative result (highest p value) of the two and considered the less conservative result as a sensitivity analysis.49 We adjusted for zero-event cells using treatment-arm continuity correction. For trials with multiple relevant experimental groups, we divided the number of events and sample size of the control group for dichotomous outcomes and divided the sample size and kept the mean and SD of the control group for continuous outcomes. If the data could not be equally divided due to an odd number of events, we drew lots to decide which comparison would be favoured. We used the statistical software Stata V.17 to analyse the data.52 Trial sequential analysis was used to control for random errors by estimating the diversity-adjusted required information size, which is the number of participants needed in a meta-analysis to detect or reject a certain intervention effect.53–61 To assess clinical significance, we used the lowest estimate based on various methods to determine the minimal important difference as detailed by Hengartner and Plöderl.17 The lowest empirically derived threshold of clinical significance is three points on the HDRS, which was predefined in our protocol.27 However, it has previously been questioned whether the true minimal important difference is in fact closer to seven points.62 We used Grading Recommendations Assessment Development Evaluation (GRADE) to assess the certainty of evidence.63–65
Differences between the protocol and the review
Suicidal ideation was predefined as a continuous scale, but the outcome was reported as a dichotomous outcome, and we therefore analysed it accordingly.
Findings
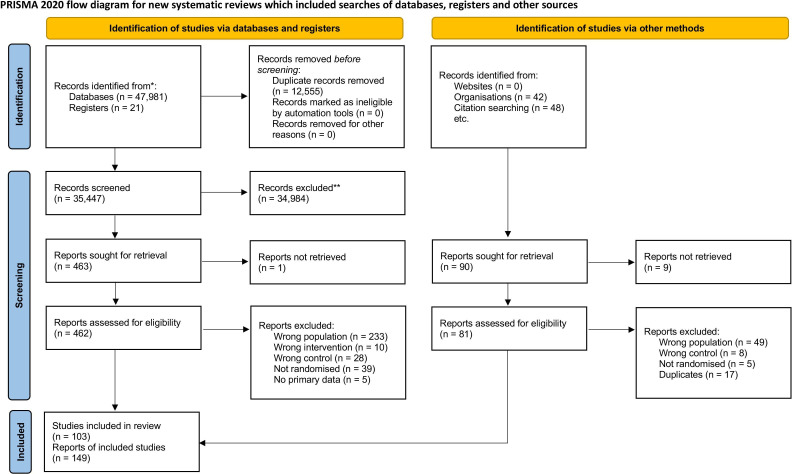
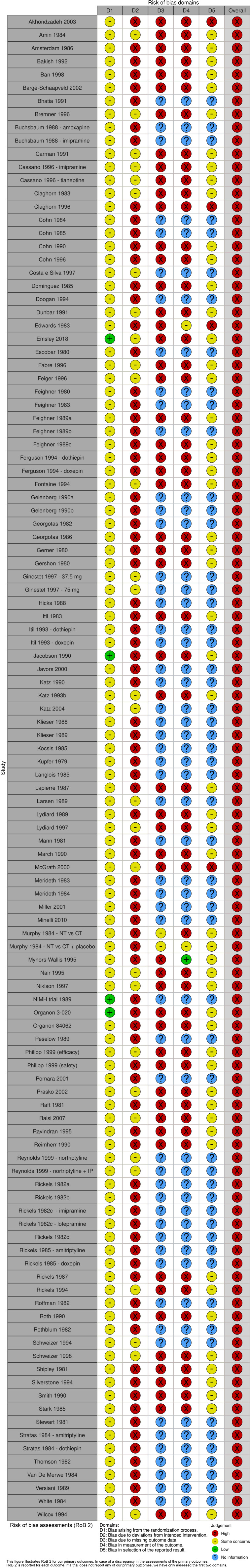
A total of 103 trials randomising 10 590 participants were included (figure 1).66–223 Most trials (92/103) included both men and women between 18 and 65 years of age with a primary diagnosis of major depressive disorder (online supplemental table S1). Ten trials only included elderly participants (defined by trialists as above 50–65 years).90 110 144 146 159 170 196 211 213 214 The mean HDRS baseline scores ranged from 17.4 to 45.5 (online supplemental table S1). Both the experimental and the control participants in eight trials also received a co-intervention, such as psychotherapy or other drugs.78 83 103 131 187 198 211 215 The included trials assessed the effects of different tricyclic antidepressants: imipramine (50 trials),31 69 84 87 90 98–103 114 117–119 121 123–127 133–136 142 146 150 152–155 163 164 166–170 187 208 210 212–214 217 218 224 amitriptyline (31 trials),66–68 70–72 74–80 82 83 85–89 91–96 151 221–223 225 nortriptyline (8 trials),110 131 159 179 196 198 209 211 desipramine (6 trials),104 130 143 177 178 207 dothiepin (4 trials),73 93 112 137 tianeptine (4 trial),117 144 174 201 doxepin (3 trials),88 112 137 clomipramine (2 trials),81 149 amoxapine (1 trial),150 cianopramine (1 trial),226 lofepramine (1 trial)224 and maprotiline (1 trial).180 Inert placebos were used in 102 trials, while only one trial used ‘active placebo’ as control intervention.198 All trials were assessed at overall high risk of bias (figure 2). Ninety-four trials (91%) were at risk of for-profit bias (online supplemental table S1). Most trials did not adequately report the proportion of participants with missing data at follow-up, and it was therefore not possible to perform ‘best-worst/worst-best’ sensitivity analyses.
Figure 1.
PRISMA flow diagram. From: Page et al.242
Figure 2.
Risk of bias (RoB) assessments.
Eleven of the included trials assessed outcomes after an extended period of treatment.67 69 72 90 98 119 134 135 164 211 220 However, in these trials it was either optional to extend the treatment and follow-up period or there were no available data. The trial authors excluded participants from the follow-ups in the extended phase, if they did not wish to extend their treatment, and we therefore chose to exclude these potentially biased data in our analyses. Four other trials assessed outcomes up to 18 months after treatment completion, but no relevant outcomes were reported at these time points.104 180 184 200
Primary outcomes
Hamilton Depression Rating Scale, 17 items
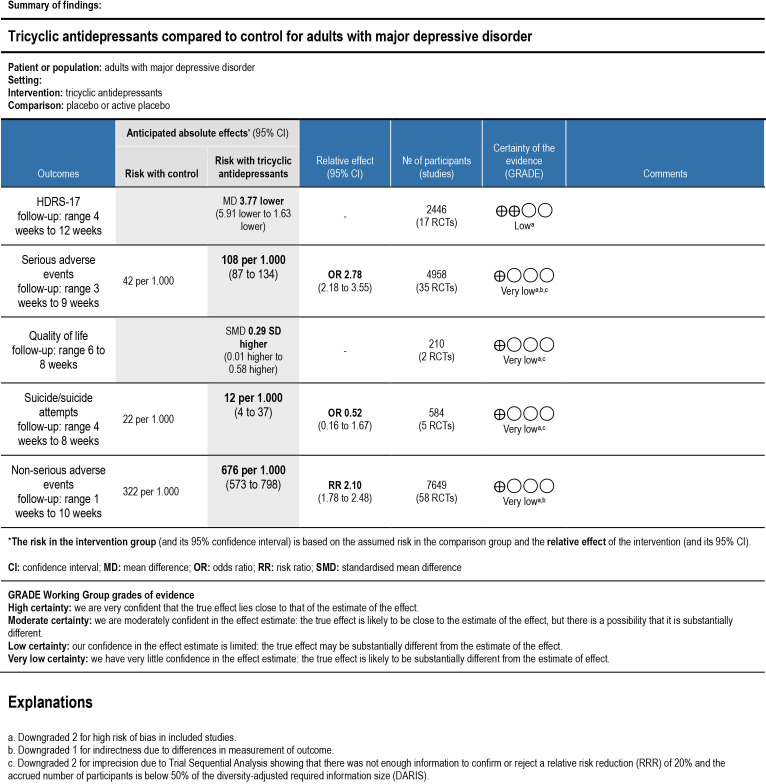
Only 17 trials reported results on HDRS-17.69 82–84 86 91 101 127 130 137 144 152 198 215 221 222 All trials only assessed outcomes at the end of the treatment period, that is, from 4 to 12 weeks after randomisation. Meta-analysis showed evidence of a beneficial effect of tricyclic antidepressants (mean difference (MD) −3.77 HDRS-17 points; 95% CI −5.91 to −1.63; p<0.01; 17 trials; Bayes factor: 0.003) (online supplemental figure S1). Visual inspection of the forest plot and statistical tests (τ=4.4; I2=91.6%) indicated substantial heterogeneity. When an outlier with a relatively large difference between the HDRS-17 baseline scores (tricyclic group: 38.5, placebo group: 44.2) was removed,91 meta-analysis showed evidence of a beneficial effect of tricyclic antidepressants (MD −3.16 HDRS-17 points; 95% CI −4.29 to −2.04; p<0.01; τ=1.9; I2=67.4%; 16 trials) (online supplemental figure S2). Visual inspection of the funnel plot did not show clear signs of asymmetry (online supplemental figure S3). Trial sequential analysis showed that we had enough information to confirm that tricyclic antidepressants reduced the HDRS-17 score (online supplemental figure S4). This outcome result was assessed as overall high risk of bias, and the certainty of the evidence was low (figure 3).
Figure 3.
Summary of findings table. HDRS, Hamilton Depression Rating Scale; GRADE, Grading Recommendations Assessment Development Evaluation; RCT, randomised clinical trial.
Test of interaction comparing trials using ‘active placebo’ to trials using inert placebo showed evidence of a difference (p=0.01) (online supplemental figure S5). When the trial using ‘active placebo’ (atropine and phenobarbital) was analysed separately, meta-analysis showed no evidence of an effect of tricyclic antidepressants (MD 2.47; 95% CI −2.07 to 7.01; p=0.29; 1 trial). When the subgroup of trials using inert placebo was analysed separately, meta-analysis showed evidence of a beneficial effect of tricyclic antidepressants (MD −4.08; 95% CI −6.22 to −1.93; p<0.01; 16 trials).
Tests of interaction comparing the effects of different tricyclic antidepressants (p=0.15), use of placebo washout period (p=0.09) and age groups (p=0.98) showed no evidence of differences (online supplemental figures S6–S8). The remaining predefined subgroup analyses were not possible to perform due to lack of relevant data.
Serious adverse events
None of the included trials reported serious adverse events according to the ICH-GCP definition,31 and only four trials with few randomised participants and very few events assessed serious adverse events as a composite outcome (online supplemental file 3).
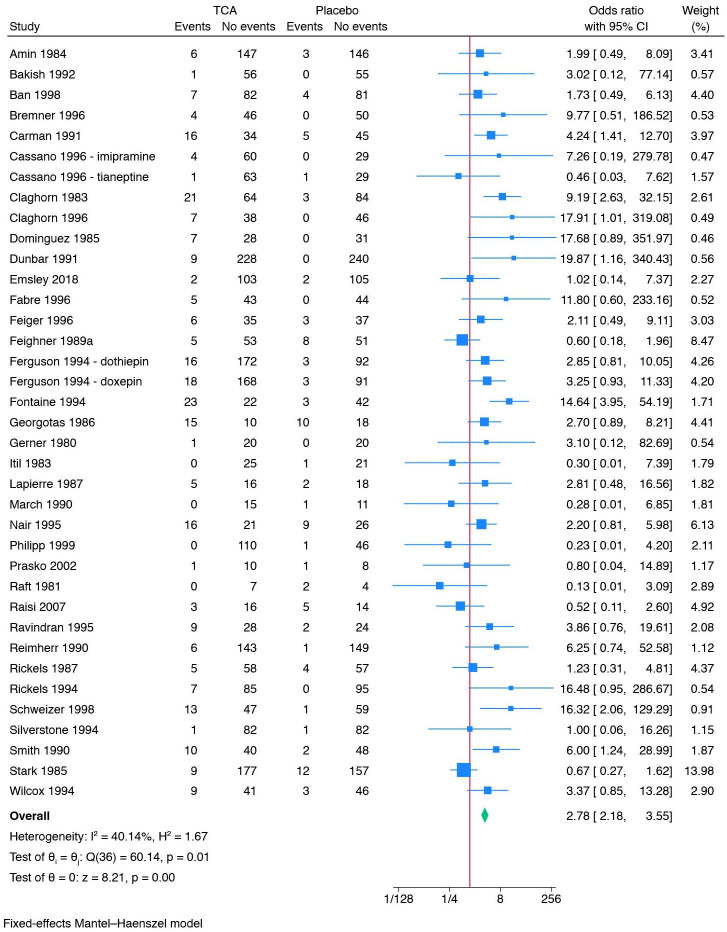
Thirty-five trials reported data that we categorised as serious adverse events based on the ICH-GCP definition (online supplemental table 2).67 71 72 84–86 90 92 96 98–101 103 104 110 117 118 124 125 131 137 138 142 144 146 153 154 159 163 167 170 202 207 218 220 The trial using ‘active placebo’ was not included in this meta-analysis. All trials only assessed outcomes at the end of the treatment period, that is, from 3 to 9 weeks after randomisation. A total of 268/2661 (10.1%) experimental participants had one or more serious adverse events compared with 96/2297 (4.2%) control participants. Meta-analysis showed evidence of a harmful effect of tricyclic antidepressants on serious adverse events (OR 2.78; 95% CI 2.18 to 3.55; p<0.01; 35 trials; Bayes factor: 1.72 E-05) (figure 4). Visual inspection of the forest plot and statistical tests (I2=40.1%) indicated heterogeneity that could not be resolved. Trial sequential analysis showed that we did not have enough information to confirm or reject the hypothesis that tricyclic antidepressants increased the risk of serious adverse events with a relative risk reduction of 20% (online supplemental figure S9). This outcome result was assessed as overall high risk of bias, and the certainty of the evidence was very low (figure 3).
Figure 4.
Meta-analysis of tricyclic antidepressants versus placebo on serious adverse events.
Test of interaction comparing trials at risk of for-profit bias to trials without risk of for-profit bias showed evidence of a difference (p<0.01) (online supplemental figure S10). When the subgroup of trials at risk of for-profit bias was analysed separately, meta-analysis showed evidence of a harmful effect of tricyclic antidepressants (OR 3.01; 95% CI 2.34 to 3.88; p<0.01; 32 trials). When the subgroup of trials without risk of for-profit bias was analysed separately, meta-analysis showed no evidence of a difference (OR 0.43; 95% CI 0.12 to 1.51; p=0.19; 3 trials).
Tests of interaction comparing the effects of different tricyclic antidepressants (p=0.28), age groups (p=0.70) and use of placebo washout period (p=0.55) showed no evidence of differences (online supplemental figures S11–S13). The remaining predefined subgroup analyses were not possible to perform due to lack of relevant data.
When each specific serious adverse event was analysed separately, 5/15 meta-analyses showed evidence of a harmful effect of tricyclic antidepressants on: hypotension (risk ratio (RR) 3.31; 95% CI 1.93 to 5.68; p<0.01; τ=0.5; I2=43.6%; 10 trials; number needed to harm (NNH): 8 (111/636)) (online supplemental figure S14); urinary retention (RR 6.07; 95% CI 1.66 to 22.19; p<0.01; τ=0.9; I2=38.2%; five trials; NNH: 8 (36/266)) (online supplemental figure S15); amblyopia (RR 3.32; 95% CI 1.94 to 5.66; p<0.01; τ=0.2; I2=6.0%; five trials; NNH: 11 (73/574)) (online supplemental figure S16); sexual dysfunction (RR 3.50; 95% CI 1.29 to 9.48; p=0.01; τ=0.6; I2=16.8%; eight trials; NNH: 31 (25/651)) (online supplemental figure S17); and taste alteration (RR 4.04; 95% CI 1.23 to 13.24; p=0.02; τ=0.6; I2=19.9%; four trials; NNH: 35 (26/677)) (online supplemental figure S18). The 10 remaining meta-analyses showed no evidence of differences (online supplemental table S3 and figures S19–S28).
Quality of life
Only two trials reported mean scores and SD for quality of life.69 84 Quality of life was assessed using either a Visual Analogue Scale69 or the mental component scale of the Short Form 36.84 Both trials only assessed outcomes at the end of the treatment period, that is, from 6 to 8 weeks after randomisation. One trial randomised 63 participants, and our t-test showed no evidence of a difference on quality of life (t(57) = 0.95, p=0.35).69 The other trial randomised 157 participants, and our t-test showed no evidence of a difference on quality of life (t(155) = 1.81, p=0.07).84 These results were assessed as overall high risk of bias, and the certainty of the evidence was very low (figure 3).
Secondary outcomes
Suicides or suicide attempts
Only 5 of the 103 trials reported on suicides or suicide attempts.84 101 117 125 All trials only assessed outcomes at the end of the treatment period, that is, from 4 to 8 weeks after randomisation. A total of 3/361 (0.8%) experimental participants had a suicide or suicide attempts compared with 5/223 (2.2%) control participants. Meta-analysis showed no evidence of a difference between tricyclic antidepressants and placebo on suicides or suicide attempts (OR 0.52; 95% CI 0.16 to 1.67; p=0.27; five trials; Bayes factor: 0.71) (online supplemental figure S29). Visual inspection of the forest plot and statistical tests (I2=0.0%) indicated no clear signs of heterogeneity. Trial sequential analysis showed that we did not have enough information to confirm or reject the hypothesis that tricyclic antidepressants reduced the risk of suicides or suicide attempts with a relative risk reduction of 20% (no graph produced). This outcome result was assessed as overall high risk of bias, and the certainty of the evidence was very low (figure 3).
Non-serious adverse events
Fifty-eight trials reported on non-serious adverse events.66 67 69–73 76 79 81 82 84–87 89 90 92 96 98–102 104 110 114 117–119 121 124 125 127 129 131 135 137 142 144 146 153 154 159 163 164 167 170 171 174 201 202 207 209 213 218 220 223 224 226 Trials using ‘active placebo’ were not included in this meta-analysis. All trials only assessed outcomes at the end of the treatment period, that is, from 1 to 10 weeks after randomisation. A total of 2595/4103 (63.2%) experimental participants had one or more non-serious adverse events compared with 1141/3546 (32.2%) control participants. Meta-analysis showed evidence of a harmful effect of tricyclic antidepressants on non-serious adverse events (RR 2.10; 95% CI 1.78 to 2.48; p<0.01; 58 trials; Bayes factor: 6.32 E-08) (figure 5). Visual inspection of the forest plot and statistical tests (τ=0.6; I2=93.5%) indicated heterogeneity that could not be resolved. Trial sequential analysis showed that we had enough information to confirm that tricyclic antidepressants increased the risk of non-serious adverse events (online supplemental figure S30). This outcome result was assessed as overall high risk of bias and the certainty of the evidence was very low (figure 3).
Figure 5.
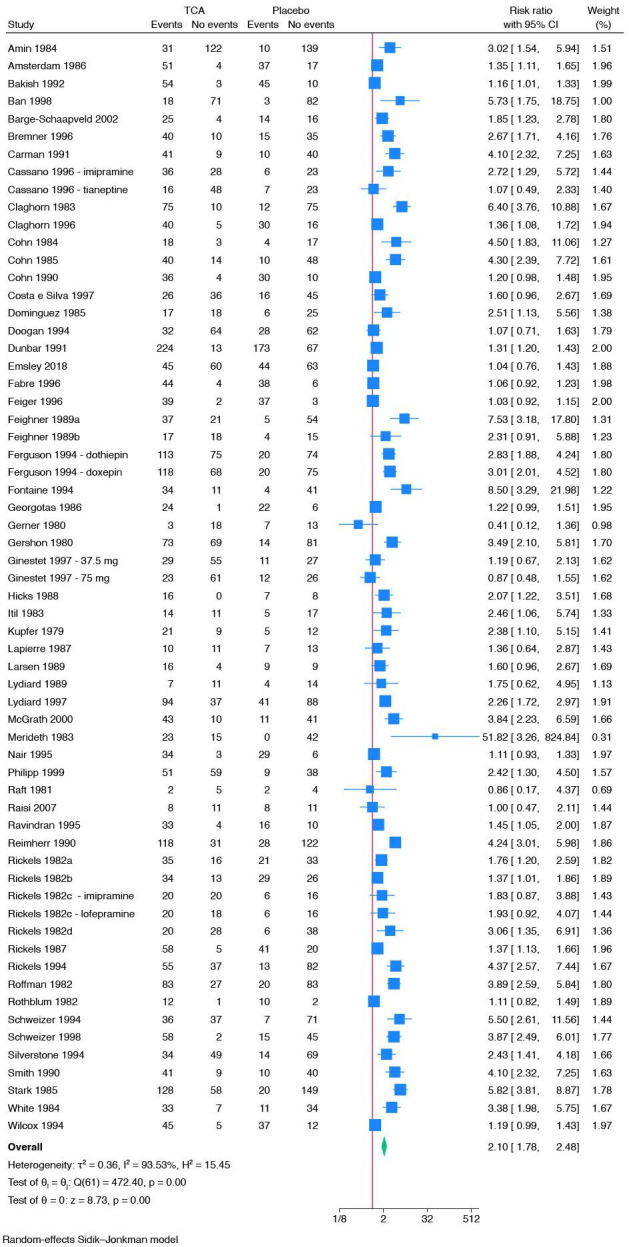
Meta-analysis of tricyclic antidepressants (TCA) versus placebo on non-serious adverse events.
One trial used another drug, citalopram, as a co-intervention. Test of interaction comparing the effects of drug co-interventions versus no drug co-intervention showed no evidence of a difference (p=0.053) (online supplemental figure S31).
When each specific non-serious adverse event was analysed separately, 23/36 meta-analyses showed evidence of a harmful effect of tricyclic antidepressants on individual non-serious adverse events: dry mouth (45 trials), constipation (38 trials), dizziness (34 trials), somnolence (33 trials), tremor (28 trials), sweating (21 trials), blurred vision (20 trials), asthenia (20 trials), nervousness (14 trials), tachycardia (14 trials), dyspepsia (11 trials), weight gain (8 trials), paraesthesia (7 trials), confusion (7 trials), anticholinergic symptoms (5 trials), sedation (5 trials), increased appetite (5 trials), decreased appetite (4 trials), micturition disorder (3 trials), flushing (2 trials), abnormal dreams (2 trials), impaired urination (2 trials) and urinary hesitancy (2 trials). The 10 non-serious adverse events with the lowest NNH were dry mouth (RR 3.43; 95% CI 2.87 to 4.10; p<0.01; τ=0.5; I2=72.1%; 45 trials; NNH: 2 (1863/3399)) (online supplemental figure S32); anticholinergic symptoms (RR 2.35; 95% CI 1.46 to 3.78; p<0.01; τ=0.5; I2=79.0%; 5 trials; NNH: 3 (184/297)) (online supplemental figure S33); somnolence (RR 2.65; 95% CI 2.20 to 3.21; p<0.01; τ=0.4; I2=55.9%; 33 trials; NNH: 4 (919/2616)) (online supplemental figure S34); sedation (RR 1.67; 95% CI 1.08 to 2.58; p=0.02; τ=0.4; I2=49.1%; 5 trials; NNH: 7 (98/301)) (online supplemental figure S35); dizziness (RR 2.37; 95% CI 1.87 to 3.01; p<0.01; τ=0.5; I2=56.6%; 34 trials; NNH: 7 (584/2753)) (online supplemental figure S36); constipation (RR 2.81; 95% CI 2.16 to 3.65; p<0.01; τ=0.6; I2=58.6%; 38 trials; NNH: 7 (617/3082)) (online supplemental figure S37); sweating (RR 3.64; 95% CI 2.41 to 5.50; p<0.01; τ=0.6; I2=42.5%; 21 trials; NNH: 8 (230/1563)) (online supplemental figure S38); tremor (RR 4.70; 95% CI 3.02 to 7.30; p<0.01; τ=0.8; I2=47.1%; 28 trials; NNH: 9 (300/2321)) (online supplemental figure S39); blurred vision (RR 2.96; 95% CI 2.21 to 3.96; p<0.01; τ=0.2; I2=14.7%; 19 trials; NNH: 10 (216/1485)) (online supplemental figure S40) and flushing (RR 5.86; 95% CI 1.33 to 25.72; p=0.02; τ=0.7; I2=41.0%; 2 trials; NNH: 10 (26/231)) (online supplemental figure S41). Two meta-analyses showed evidence of a beneficial effect of tricyclic antidepressants on individual non-serious adverse events: diarrhoea (RR 0.46; 95% CI 0.29 to 0.74; p<0.01; τ=0.4; I2=25.0%; 13 trials; number needed to treat (NNT): 19 (35/895)) (online supplemental figure S42) and infection (RR 0.41; 95% CI 0.19 to 0.89; p=0.02; τ=0.1; I2=3.9%; 3 trials; NNT: 21 (9/279)) (online supplemental table S4 and figure S43). The remaining meta-analyses are reported in the online supplemental material (online supplemental table S5 and figures S44–S67). Please see online supplemental file 4 for the list of non-serious adverse events combined for meta-analyses.
The results of the remaining exploratory outcomes, sensitivity analyses and prediction intervals are reported in online supplemental file 3 and online supplemental figures S68–S133.
Discussion
We conducted a systematic review assessing the beneficial and harmful effects of tricyclic antidepressants for adults with major depressive disorder. A total of 103 placebo-controlled trials randomising 10 590 participants were included. In comparison, the network meta-analysis by Cipriani et al19 included 36 trials assessing the effects of tricyclic antidepressants versus placebo since they only assessed amitriptyline and clomipramine. All present outcome results were at overall high risk of bias and the certainty of evidence was very low or low, particularly due to lack of information, missing data, lack of blinding of outcome assessors, risk of unblinding due to adverse effects, inappropriate analysis methods and poor reporting. All trials only assessed outcomes at the end of the treatment period at a maximum of 12 weeks after randomisation. Meta-analysis and trial sequential analysis showed that tricyclic antidepressants reduced depressive symptoms more than placebo, but the certainty was low. Meta-analysis showed evidence of a harmful effect of tricyclic antidepressants compared with placebo on serious adverse events, but the required information size was not reached and the certainty was very low. The serious adverse events with the lowest NNH were hypotension, urinary retention, amblyopia, sexual dysfunction and taste alteration. Only 2 out of 103 trials reported on quality of life, and t-tests showed no evidence of an intervention effect. Meta-analysis and trial sequential analysis showed that we did not have enough information to confirm or reject the effects of tricyclic antidepressants on suicides or suicide attempts. Meta-analysis and trial sequential analysis showed evidence of a harmful effect of tricyclic antidepressants compared with placebo on non-serious adverse events. The non-serious adverse events with the lowest NNH were dry mouth, anticholinergic symptoms, somnolence, sedation and dizziness.
Our meta-analysis showed a mean difference between tricyclic antidepressants and placebo of −3.77 HDRS points or −3.16 HDRS points when an outlier was removed. We predefined the minimal important difference on HDRS as three points, but it has been questioned whether the true minimal important difference is in fact closer to seven points.62 Moreover, the effect was not above our minimal important difference in the one trial using an ‘active placebo’. The high risk of bias of the included trials and the low certainty of the evidence make our results inadequate to determine whether tricyclic antidepressants have a genuine and meaningful short-term antidepressant effect rather than an amplified placebo effect.
Our systematic review has several strengths. Our results are novel, as this is the first systematic review assessing all adverse effects for all tricyclic antidepressants in adults with major depressive disorder. Data on adverse effects are essential for enabling patients and clinician to make informed decisions about the use of any treatment. The predefined methodology was based on the Cochrane Handbook for Systematic Reviews of Interventions,227 PRISMA,25 trial sequential analysis,53 59 the eight-step procedure by Jakobsen et al,49 the GRADE approach,63 and risks of systematic and random errors, external validity, publication bias and heterogeneity were taken into account. We increased the statistical power by pooling all tricyclic antidepressants, and we compared the effects of different types of tricyclic antidepressants in subgroup analyses. Furthermore, we searched for both published trials and unpublished data to increase the validity of our results.227–230
Our systematic review also has limitations. First, the included trials only reported results at the end of treatment at a maximum of 12 weeks, so the long-term effects of tricyclic antidepressants are unknown. There is a need for trials with long-term follow-up to assess the benefits and harms since, for example, half of patients on antidepressants in the UK and 70% of patients in the USA have used them for more than 2 years.231 232 This is particularly pertinent for medications that are associated with tolerance and withdrawal effects, which tend to show diminishing effects over time.233 Second, all included trials were assessed at overall high risk of bias particularly driven by risk of bias due to missing data, lack of blinding of outcome assessors, risk of unblinding due to adverse effects, inappropriate analysis methods and the reporting of the included trials was generally poor. The reporting and assessment of adverse events were especially inadequate. None of the trials assessed adverse events based on the ICH-GCP guidelines,31 and serious adverse events were generally not systematically assessed. Studies have shown that the adverse effects are generally under-reported in published trials compared with unpublished data, and we therefore aimed to include unpublished data.227–230 However, in spite of searching systematically for unpublished data, we were only able to identify unpublished data for one trial.220 Our results are therefore prone to overestimation of benefits and underestimation of harms.234–241 Third, only five of the included trials reported on suicides or suicide attempts, and there was not enough information to confirm or reject the effects of tricyclic antidepressants on suicides or suicide attempts. This is particularly problematic since major depressive disorder is associated with increased risks of suicidal behaviour.3–5 There is a need for larger trials at low risk of bias to assess the risks of suicides and suicide attempts. Fourth, only two trials had publicly available protocols or trial registrations, and the certainty of the evidence was very low or low for all outcome results. Fifth, we planned several outcome comparisons, which increased the risk of type I errors. To control the risks of random errors, we adjusted our threshold for significance according to the number of primary and secondary outcomes, but we did not adjust the thresholds for significance according to the total number of comparisons, including exploratory outcomes, subgroup analyses and sensitivity analyses. Sixth, due to poor reporting of the tricyclic antidepressant doses used in the included trials, it was not possible to define meaningful dose subgroups to compare the effects of different doses. Seventh, since we only identified one trial using ‘active placebo’, we could not adequately assess whether the nature of control intervention impacted results. Eighth, we included one trial using citalopram as a co-intervention, which may lead to different RRs for adverse events compared with other trials, but we assessed the potential differences with subgroup analyses. Ninth, we did not test the inter-rater reliability for our RoB 2 assessments. Tenth, since the included trials did not report serious adverse events according to the ICH-GCP definition and because the definition of serious adverse events was unclear, it was necessary to make a subjective assessment of the severity of the adverse events to decide if each event should be classified as a serious adverse event. However, the subjective assessments may be inaccurate as they rely on the specific adverse events chosen to be reported by the trialists—other serious adverse events might have occurred that the trialists did not assess or report. The information provided by the trialists about specific adverse events was often sparse (ie, adverse events were often only reported in tables and there was rarely information about the patients’ specific events). Hence, the present results presumably underestimate the harmful effects of tricyclic antidepressants. A subjective assessment of adverse events based on such information is therefore likely to be incomplete, but nevertheless, important data on adverse effects would not be available without this process. We believe that the present analysis of serious adverse events, a critical outcome of any drug trial, provides useful information regarding the adverse effects of tricyclic antidepressants, and we have assessed serious adverse events using this methodology in several systematic reviews for over a decade.36–47 Still, the above-mentioned limitations should be considered when interpreting our results.
Conclusions and clinical implications
The long-term effects of tricyclic antidepressants and the effects on quality of life and suicides or suicide attempts are unknown. Short-term results suggest that tricyclic antidepressants may reduce depressive symptoms while also increasing the risks of adverse events, but these results were based on low and very low certainty evidence. It is a cause for concern that there are no data on the long-term adverse effects of tricyclic antidepressants given that so many people use these drugs for several years.
Acknowledgments
We thank Sarah Louise Klingenberg (Information Specialist, The Cochrane Hepato-Biliary Group, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen University Hospital – Rigshospitalet, Denmark) for the help with developing the search strategy. We thank Kevin Han Yuan for help with translation.
Footnotes
Contributors: CBK, SJ, FS, JM, MAH, MH, IK, MB, CG and JCJ contributed to the conceptualisation and design of the study. CBK and PF screened studies for inclusion. CBK, JJP, PF, SJ, FS, ATK and MB extracted data. CBK and JJP analysed data. CBK and JCJ wrote the original draft. All authors commented and approved the final manuscript. CBK and JCJ are the guarantors. The guarantors had full access to all the data in the study, take responsibility for the integrity of the data and the accuracy of the data analyses, and had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The Copenhagen Trial Unit, Centre for Clinical Intervention Research employed CBK, JPP, PF, SJ, CG and JCJ for parts of this study.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; MAH is a co-applicant and member of the DSMB of the RELEASE trial in Australia, funded by the Medical Research Future Fund (MRFF). MAH is co-founder and consultant for Outro Health, a digital clinic helping patients to stop unnecessary antidepressant medication. MAH has been paid honoraria by several NHS Trusts for grand rounds presentations, and by Salomon’s University and the University of Washington. MAH is a member of the Critical Psychiatry Network and the International Institute of Psychiatric Drug Withdrawal (IIPDW). JM is a co-investigator on REDUCE (programme grant studying discontinuation of antidepressants) and Chief Investigator on RADAR (programme grant to explore antipsychotic reduction and discontinuation). JM has been paid honoraria by University of Basel, Alberta Psychiatric Association, and Case Western University. JM receives royalties from Palgrave Macmillan and PCCS Books for three books about psychiatric drugs. JM is a co-chair person (unfunded position) of Critical Psychiatry Network. MPH receives royalties from Palgrave Macmillan for a book about antidepressants; no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.American Psychiatric Association . Diagnostic and statistical Manual of mental disorders. In: Diagnostic and statistical manual of mental disorders (DSM-5®). Washington DC: American Psychiatric Publishing, 2013. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 2.Swann T. “'anarchist Technologies': anarchism, cybernetics and mutual aid in community responses to the COVID-19 crisis”. Organization (Lond) 2023;30:193–209. 10.1177/13505084221090632 Available: https://www.who.int/news-room/fact-sheets/detail/depression [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity survey. Arch Gen Psychiatry 1999;56:617–26. 10.1001/archpsyc.56.7.617 [DOI] [PubMed] [Google Scholar]
- 4.Qin P. The impact of psychiatric illness on suicide: differences by diagnosis of disorders and by sex and age of subjects. J Psychiatr Res 2011;45:1445–52. 10.1016/j.jpsychires.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and Unipolar disorders relative to subjects with other axis I disorders. Biol Psychiatry 1996;39:896–9. 10.1016/0006-3223(95)00295-2 [DOI] [PubMed] [Google Scholar]
- 6.Bachmann CJ, Aagaard L, Burcu M, et al. Trends and patterns of antidepressant use in children and adolescents from five Western countries, 2005-2012. Eur Neuropsychopharmacol 2016;26:411–9. 10.1016/j.euroneuro.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Kataoka Y, Ostinelli EG, et al. National prescription patterns of antidepressants in the treatment of adults with major depression in the US between 1996 and 2015: A population representative survey based analysis. Front Psychiatry 2020;11:35. 10.3389/fpsyt.2020.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chee K-Y, Tripathi A, Avasthi A, et al. International study on antidepressant prescription pattern at 40 major psychiatric institutions and hospitals in Asia: A 10-year comparison study. Asia Pac Psychiatry 2015;7:366–74. 10.1111/appy.12176 [DOI] [PubMed] [Google Scholar]
- 9.Hoefler R, Galvão TF, Ribeiro-Vaz I, et al. Trends in Brazilian market of antidepressants: A five-year Dataset analysis. Front Pharmacol 2022;13:893891. 10.3389/fphar.2022.893891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhi GS, Acar M, Kouhkamari MH, et al. Antidepressant prescribing patterns in Australia. BJPsych Open 2022;8:e120. 10.1192/bjo.2022.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soleymani F, Taheri F, Roughead E, et al. Pattern of antidepressant utilization and cost in Iran from 2006 to 2013 in comparison with other countries. J Epidemiol Glob Health 2018;8:213–9. 10.2991/j.jegh.2018.06.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz I, Serna C, Real J, et al. Comparison of the consumption of antidepressants in the immigrant and native populations in a Spanish health region: an observational study. BMC Public Health 2010;10:255. 10.1186/1471-2458-10-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence . Depression in adults: treatment and management. 2022. Available: https://www.nice.org.uk/guidance/ng222 [PubMed]
- 14.Malhi GS, Bell E, Bassett D, et al. The 2020 Royal Australian and New Zealand college of psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 2021;55:7–117. 10.1177/0004867420979353 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . World health organization model list of essential medicines, 22st list. 2021. Available: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists
- 16.Jakobsen JC, Gluud C, Kirsch I. Should antidepressants be used for major depressive disorder BMJ Evid Based Med 2020;25:130. 10.1136/bmjebm-2019-111238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengartner MP, Plöderl M. Estimates of the minimal important difference to evaluate the clinical significance of antidepressants in the acute treatment of moderate-to-severe depression. BMJ Evid Based Med 2022;27:69–73. 10.1136/bmjebm-2020-111600 [DOI] [PubMed] [Google Scholar]
- 18.Moncrieff J, Kirsch I. Efficacy of antidepressants in adults. BMJ 2005;331:155–7. 10.1136/bmj.331.7509.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357–66. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arroll B, Macgillivray S, Ogston S, et al. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med 2005;3:449–56. 10.1370/afm.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa T, McGuire H, Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane Database Syst Rev 2003;2003:CD003197. 10.1002/14651858.CD003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leucht C, Huhn M, Leucht S. Amitriptyline versus placebo for major depressive disorder. Cochrane Database Syst Rev 2012;12:CD009138. 10.1002/14651858.CD009138.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guaiana G, Barbui C, Hotopf M. Amitriptyline for depression. Cochrane Database Syst Rev 2007:CD004186. 10.1002/14651858.CD004186.pub2 [DOI] [PubMed] [Google Scholar]
- 24.PRISMA-P Group, Moher D, Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate Healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for systematic reviews of interventions. Cochrane 2022. Available: www.training.cochrane.org/handbook [Google Scholar]
- 27.Jørgensen CK, Juul S, Siddiqui F, et al. “Tricyclic antidepressants versus 'active placebo', placebo or no intervention for adults with major depressive disorder: a protocol for a systematic review with meta-analysis and trial sequential analysis”. Syst Rev 2021;10:227. 10.1186/s13643-021-01789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . International Statistical Classification of Diseases and Related Health Problems (ICD). 2021. Available: https://www.who.int/standards/classifications/classification-of-diseases [Google Scholar]
- 29.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2019. 10.1002/9781119536604 [DOI] [Google Scholar]
- 30.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 31.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . ICH Harmonised guideline: integrated Addendum to ICH E6(R1): guideline for good clinical practice (ICH-GCP)2015; step 2 version. n.d. Available: https://ichgcp.net/da
- 32.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio, TX: Psychological Corporation, 1996: 490–8. [Google Scholar]
- 34.Timmerby N, Andersen JH, Søndergaard S, et al. A systematic review of the Clinimetric properties of the 6-item version of the Hamilton depression rating scale (HAM-D6). Psychother Psychosom 2017;86:141–9. 10.1159/000457131 [DOI] [PubMed] [Google Scholar]
- 35.López-Pina JA, Sánchez-Meca J, Rosa-Alcázar AI. The Hamilton rating scale for depression: A meta-analytic reliability generalization study. Int J Clin Health Psychol 2009;9:143–59. [Google Scholar]
- 36.Holgersson J, Ceric A, Sethi N, et al. Fever therapy in febrile adults: systematic review with meta-analyses and trial sequential analyses. BMJ 2022;378:e069620. 10.1136/bmj-2021-069620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakobsen JC, Nielsen EE, Feinberg J, et al. Direct-acting Antivirals for chronic hepatitis C. Cochrane Database Syst Rev 2017;2017. 10.1002/14651858.CD012143.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakobsen JC, Nielsen EE, Koretz RL, et al. Do direct acting Antivirals cure chronic hepatitis C. BMJ 2018;361:k1382. 10.1136/bmj.k1382 [DOI] [PubMed] [Google Scholar]
- 39.Juul S, Nielsen EE, Feinberg J, et al. Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (the LIVING project). PLoS Med 2020;17:e1003293. 10.1371/journal.pmed.1003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maagaard M, Barbateskovic M, Andersen‐Ranberg NC, et al. Dexmedetomidine for the prevention of delirium in adults admitted to the intensive care unit or post-operative care unit: A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Acta Anaesthesiol Scand 2023;67:382–411. 10.1111/aas.14208 Available: https://onlinelibrary.wiley.com/toc/13996576/67/4 [DOI] [PubMed] [Google Scholar]
- 41.Maagaard M, Nielsen EE, Sethi NJ, et al. Ivabradine added to usual care in patients with heart failure: a systematic review with meta-analysis and trial sequential analysis. BMJ Evid Based Med 2022;27:224–34. 10.1136/bmjebm-2021-111724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feinberg J, Nielsen EE, Gluud C, et al. Cochrane corner: drug-Eluting Stents versus bare-metal Stents for acute coronary syndrome. Heart 2018;104:1895–7. 10.1136/heartjnl-2017-312931 [DOI] [PubMed] [Google Scholar]
- 43.Feinberg J, Nielsen EE, Greenhalgh J, et al. Drug-Eluting Stents versus bare-metal Stents for acute coronary syndrome. Cochrane Database Syst Rev 2017;8:CD012481. 10.1002/14651858.CD012481.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev 2017;2017. 10.1002/14651858.CD011598.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi NJ, Feinberg J, Nielsen EE, et al. The effects of rhythm control strategies versus rate control strategies for atrial fibrillation and atrial flutter: A systematic review with meta-analysis and trial sequential analysis. PLoS One 2017;12:e0186856. 10.1371/journal.pone.0186856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbateskovic M, Marker S, Granholm A, et al. Stress ulcer prophylaxis with proton pump inhibitors or Histamin-2 receptor antagonists in adult intensive care patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2019;45:143–58. 10.1007/s00134-019-05526-z [DOI] [PubMed] [Google Scholar]
- 47.Nielsen EE, Feinberg JB, Bu F-L, et al. Beneficial and harmful effects of Sacubitril/valsartan in patients with heart failure: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Open Heart 2020;7:e001294. 10.1136/openhrt-2020-001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keus F, Wetterslev J, Gluud C, et al. Evidence at a glance: error matrix approach for Overviewing available evidence. BMC Med Res Methodol 2010;10:90. 10.1186/1471-2288-10-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakobsen JC, Wetterslev J, Winkel P, et al. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 2014;14:120. 10.1186/1471-2288-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably Outperforms the standard Dersimonian-Laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341–50. 10.1002/sim.4780060325 [DOI] [PubMed] [Google Scholar]
- 52.StataCorp . Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC, 2019. [Google Scholar]
- 53.Copenhagen Trial Unit . TSA - Trial Sequential Analysis, Available: http://www.ctu.dk/tsa
- 54.Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 55.Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763–9. 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 56.Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta-analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287–98. 10.1093/ije/dyn188 [DOI] [PubMed] [Google Scholar]
- 57.Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses Int J Epidemiol 2009;38:276–86. 10.1093/ije/dyn179 [DOI] [PubMed] [Google Scholar]
- 58.Wetterslev J, Thorlund K, Brok J, et al. Estimating required information size by Quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 2009;9:86. 10.1186/1471-2288-9-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorlund K, Engstrøm J, Wetterslev J, et al. User manual for Trial Sequential Analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2017. Available: http://www.ctu.dk/tsa/files/tsa_manual.pdf [Google Scholar]
- 60.Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. an example using isoniazid Chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. J Clin Epidemiol 2010;2:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imberger G, Thorlund K, Gluud C, et al. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review. BMJ Open 2016;6:e011890. 10.1136/bmjopen-2016-011890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moncrieff J, Kirsch I. Empirically derived criteria cast doubt on the clinical significance of antidepressant-placebo differences. Contemp Clin Trials 2015;43:60–2. 10.1016/j.cct.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 63.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the. J Clin Epidemiol 2011;64:380–2. 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 64.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schünemann HJ, Best D, Vist G, et al. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. Can Med Assoc J 2003;169:677–80. [PMC free article] [PubMed] [Google Scholar]
- 66.Amsterdam JD, Case WG, Csanalosi E, et al. A double-blind comparative trial of Zimelidine, amitriptyline, and placebo in patients with mixed anxiety and depression. Pharmacopsychiatry 1986;19:115–9. 10.1055/s-2007-1017167 [DOI] [PubMed] [Google Scholar]
- 67.Bakish D, Bradwejn J, Nair N, et al. A comparison of Moclobemide, amitriptyline and placebo in depression: a Canadian Multicentre study. Psychopharmacology 1992;106:S98–101. 10.1007/BF02246248 [DOI] [PubMed] [Google Scholar]
- 68.Bhatia SC, Hsieh HH, Theesen KA, et al. Platelet Alpha-2 Adrenoreceptor activity pre-treatment and post-treatment in major depressive disorder with Melancholia. Res Commun Chem Pathol Pharmacol 1991;74:47–57. [PubMed] [Google Scholar]
- 69.Barge-Schaapveld DQCM, Nicolson NA. Effects of antidepressant treatment on the quality of daily life: an experience sampling study. J Clin Psychiatry 2002;63:477–85. 10.4088/jcp.v63n0603 [DOI] [PubMed] [Google Scholar]
- 70.Bremner JD. A double-blind comparison of org 3770, amitriptyline, and placebo in major depression. J Clin Psychiatry 1995;56:519–25. [PubMed] [Google Scholar]
- 71.Carman JS, Ahdieh H, Wyatt-Knowles E, et al. A controlled study of Mianserin in moderately to severely depressed outpatients. Psychopharmacol Bull 1991;27:135–9. [PubMed] [Google Scholar]
- 72.Claghorn J, Gershon S, Goldstein BJ. Zimeldine tolerability in comparison to amitriptyline and placebo: findings from a Multicentre trial. Acta Psychiatr Scand Suppl 1983;308:104–14. 10.1111/j.1600-0447.1983.tb11109.x [DOI] [PubMed] [Google Scholar]
- 73.Doogan DP, Langdon CJ. A double-blind, placebo-controlled comparison of sertraline and Dothiepin in the treatment of major depression in general practice. Int Clin Psychopharmacol 1994;9:95–100. 10.1097/00004850-199400920-00005 [DOI] [PubMed] [Google Scholar]
- 74.Gelenberg AJ, Wojcik JD, Falk WE, et al. Clovoxamine in the treatment of depressed outpatients: A double-blind, parallel-group comparison against amitriptyline and placebo. Compr Psychiatry 1990;31:307–14. 10.1016/0010-440x(90)90037-s [DOI] [PubMed] [Google Scholar]
- 75.Georgotas A, Krakowski M, Gershon S. Controlled trial of Zimelidine, a 5-HT reuptake inhibitor, for treatment of depression. Am J Psychiatry 1982;139:1057–8. 10.1176/ajp.139.8.1057 [DOI] [PubMed] [Google Scholar]
- 76.Hicks F, Robins E, Murphy GE. Comparison of Adinazolam, amitriptyline, and placebo in the treatment of Melancholic depression. Psychiatry Res 1988;23:221–7. 10.1016/0165-1781(88)90012-1 [DOI] [PubMed] [Google Scholar]
- 77.Katz RJ, Lott M, Landau P, et al. A clinical test of noradrenergic involvement in the therapeutic mode of action of an experimental antidepressant. Biol Psychiatry 1993;33:261–6. 10.1016/0006-3223(93)90292-l [DOI] [PubMed] [Google Scholar]
- 78.Klieser E, Lehmann E. Experimental comparison between the effect of standardized trazodone-amitriptyline and placebo treatment in vitalized depressive patients. Psychopharmacology 1988;95. 10.1007/BF00172621 [DOI] [PubMed] [Google Scholar]
- 79.Kupfer DJ, Coble PA, Rubinstein D. Changes in weight during treatment for depression. Psychosom Med 1979;41:535–44. 10.1097/00006842-197911000-00004 [DOI] [PubMed] [Google Scholar]
- 80.Langlois R, Cournoyer G, de Montigny C, et al. High incidence of Multisystemic reactions to Zimeldine. Eur J Clin Pharmacol 1985;28:67–71. 10.1007/BF00635710 [DOI] [PubMed] [Google Scholar]
- 81.Larsen JK, Holm P, Høyer E, et al. Moclobemide and Clomipramine in reactive depression. A placebo-controlled randomized clinical trial. Acta Psychiatr Scand 1989;79:530–6. 10.1111/j.1600-0447.1989.tb10299.x [DOI] [PubMed] [Google Scholar]
- 82.Lydiard RB, Stahl SM, Hertzman M, et al. A double-blind, placebo-controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. J Clin Psychiatry 1997;58:484–91. 10.4088/jcp.v58n1104 [DOI] [PubMed] [Google Scholar]
- 83.Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, et al. Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. BMJ 1995;310:441–5. 10.1136/bmj.310.6977.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Philipp M, Kohnen R, Hiller KO. Hypericum extract versus Imipramine or placebo in patients with moderate depression: randomised Multicentre study of treatment for eight weeks. BMJ 1999;319:1534–8. 10.1136/bmj.319.7224.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raft D, Davidson J, Wasik J, et al. Relationship between response to Phenelzine and MAO inhibition in a clinical trial of Phenelzine, amitriptyline and placebo. Neuropsychobiology 1981;7:122–6. 10.1159/000117841 [DOI] [PubMed] [Google Scholar]
- 86.Reimherr FW, Chouinard G, Cohn CK, et al. Antidepressant efficacy of sertraline: a double-blind, Placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. J Clin Psychiatry 1990;51:18–27. [PubMed] [Google Scholar]
- 87.Rickels K, Case WG. Trazodone in depressed outpatients. AJP 1982;139:803–6. 10.1176/ajp.139.6.803 [DOI] [PubMed] [Google Scholar]
- 88.Rickels K, Feighner JP, Smith WT. Alprazolam, amitriptyline, doxepin, and placebo in the treatment of depression. Arch Gen Psychiatry 1985;42:134–41. 10.1001/archpsyc.1985.01790250028004 [DOI] [PubMed] [Google Scholar]
- 89.Roffman M, Gould E. A double-blind comparative study of Oxaprotiline with amitriptyline and placebo in moderate depression. Current Therapeutic Research 1982;32:2247–56. [Google Scholar]
- 90.Schweizer E, Rickels K, Hassman H, et al. Buspirone and Imipramine for the treatment of major depression in the elderly. J Clin Psychiatry 1998;59:175–83. 10.4088/JCP.v59n0406 [DOI] [PubMed] [Google Scholar]
- 91.Shipley JE, Kupfer DJ, Spiker DG, et al. Neuropsychological assessment and EEG sleep in affective disorders. Biol Psychiatry 1981;16:907–18. [PubMed] [Google Scholar]
- 92.Smith WT, Glaudin V, Panagides J, et al. Mirtazapine vs. amitriptyline vs. placebo in the treatment of major depressive disorder. Psychopharmacol Bull 1990;26:191–6. [PubMed] [Google Scholar]
- 93.Stratas NE. A double-blind study of the efficacy and safety of Dothiepin hydrochloride in the treatment of major depressive disorder. J Clin Psychiatry 1984;45:466–9. [PubMed] [Google Scholar]
- 94.Thomson J, Rankin H, Ashcroft GW, et al. The treatment of depression in general practice: a comparison of L-Tryptophan, amitriptyline, and a combination of L-Tryptophan and amitriptyline with placebo. Psychol Med 1982;12:741–51. 10.1017/S0033291700049047 [DOI] [PubMed] [Google Scholar]
- 95.van de Merwe TJ, Silverstone T, Ankier SI, et al. A double-blind non-crossover placebo-controlled study between group comparison of trazodone and amitriptyline on cardiovascular function in major depressive disorder. Psychopathology 1984;17:64–76. 10.1159/000284094 [DOI] [PubMed] [Google Scholar]
- 96.Wilcox CS, Cohn JB, Katz BB, et al. A double-blind, placebo-controlled study comparing Mianserin and amitriptyline in moderately depressed outpatients. Int Clin Psychopharmacol 1994;9:271–9. 10.1097/00004850-199400940-00006 [DOI] [PubMed] [Google Scholar]
- 97.Bakish D, Wiens A, Ellis J, et al. A double-blind placebo-controlled comparison of Moclobemide and amitriptyline in the treatment of depression. Can J Psychiatry 1992;37:12–7. [PubMed] [Google Scholar]
- 98.Feiger AD. A double-blind comparison of Gepirone extended release, Imipramine, and placebo in the treatment of outpatient major depression. Psychopharmacol Bull 1996;32:659–65. [PubMed] [Google Scholar]
- 99.Fontaine R, Ontiveros A, Elie R, et al. A double-blind comparison of Nefazodone, Imipramine, and placebo in major depression. J Clin Psychiatry 1994;55:234–41. [PubMed] [Google Scholar]
- 100.Rickels K, Chung HR, Csanalosi IB, et al. Alprazolam, diazepam, Imipramine, and placebo in outpatients with major depression. Arch Gen Psychiatry 1987;44:862–6. 10.1001/archpsyc.1987.01800220024005 [DOI] [PubMed] [Google Scholar]
- 101.Silverstone T. A Multicentre comparative trial of Moclobemide, Imipramine and placebo in major depressive disorder. Int Clin Psychopharmacol 1994;9:109–14. 10.1097/00004850-199400920-00007 [DOI] [PubMed] [Google Scholar]
- 102.Feighner JP, Boyer WF, Meredith CH, et al. A placebo-controlled inpatient comparison of fluvoxamine maleate and Imipramine in major depression. Int Clin Psychopharmacol 1989;4:239–44. 10.1097/00004850-198907000-00006 [DOI] [PubMed] [Google Scholar]
- 103.Prasko J, Horacek J, Klaschka J, et al. Bright light therapy and/or Imipramine for Inpatients with recurrent non-seasonal depression. Neuro Endocrinol Lett 2002;23:109–13. [PubMed] [Google Scholar]
- 104.Ban TA, Gaszner P, Aguglia E, et al. Clinical efficacy of Reboxetine: a comparative study with desipramine, with methodological considerations. Hum Psychopharmacol Clin Exp 1998;13:S29–39. Available: http://doi.wiley.com/10.1002/(SICI)1099-1077(199802)13:1+<>1.0.CO;2-B [DOI] [Google Scholar]
- 105.Georgotas A, McCue RE, Cooper T, et al. Clinical predictors of response to antidepressants in elderly patients. Biol Psychiatry 1987;22:733–40. 10.1016/0006-3223(87)90205-8 [DOI] [PubMed] [Google Scholar]
- 106.Georgotas A, McCue RE, Friedman E, et al. Response of depressive symptoms to nortriptyline, Phenelzine and placebo. Br J Psychiatry 1987;151:102–6. 10.1192/bjp.151.1.102 [DOI] [PubMed] [Google Scholar]
- 107.Georgotas A, McCue RE, Friedman E, et al. Electrocardiographic effects of nortriptyline, Phenelzine, and placebo under optimal treatment conditions. Am J Psychiatry 1987;144:798–801. 10.1176/ajp.144.6.798 [DOI] [PubMed] [Google Scholar]
- 108.Georgotas A, McCue RE, Friedman E, et al. A placebo-controlled comparison of the effect of nortriptyline and Phenelzine on orthostatic hypotension in elderly depressed patients. J Clin Psychopharmacol 1987;7:413–6. [PubMed] [Google Scholar]
- 109.Georgotas A, McCue RE, Reisberg B, et al. The effects of mood changes and antidepressants on the cognitive capacity of elderly depressed patients. Int Psychogeriatr 1989;1:135–43. 10.1017/s1041610289000141 [DOI] [PubMed] [Google Scholar]
- 110.Georgotas A, McCue RE, worth WH, et al. Comparative efficacy and safety of Maois versus Tcas in treating depression in the elderly. Biological Psychiatry 1986;21:1155–66. 10.1016/0006-3223(86)90222-2 [DOI] [PubMed] [Google Scholar]
- 111.Georgotas A, Stokes P, McCue RE, et al. The usefulness of DST in predicting response to antidepressants: A placebo-controlled study. Journal of Affective Disorders 1986;11:21–8. 10.1016/0165-0327(86)90055-8 [DOI] [PubMed] [Google Scholar]
- 112.Itil TM, Arikan MK, Itil KZ, et al. Clinical CEEG/DBM findings with a new antidepressant: Dothiepin. Integr Psychiatry 1992;8:241–51. [Google Scholar]
- 113.Brady KT, Lydiard RB, Kellner CH, et al. A comparison of the effects of Imipramine and fluvoxamine on the thyroid axis. Biol Psychiatry 1994;36:778–9. 10.1016/0006-3223(94)90092-2 [DOI] [PubMed] [Google Scholar]
- 114.Lydiard RB, Laird LK, Morton WA, et al. Fluvoxamine, Imipramine, and placebo in the treatment of depressed outpatients: effects on depression. Psychopharmacol Bull 1989;25:68–70. [PubMed] [Google Scholar]
- 115.Johnson MR, Bruce Lydiard R, Alexander Morton W, et al. Effect of fluvoxamine, Imipramine and placebo on catecholamine function in depressed outpatients. Journal of Psychiatric Research 1993;27:161–72. 10.1016/0022-3956(93)90004-L [DOI] [PubMed] [Google Scholar]
- 116.Laird LK, Lydiard RB, Morton WA, et al. Cardiovascular effects of Imipramine, fluvoxamine, and placebo in depressed outpatients. J Clin Psychiatry 1993;54:224–8. [PubMed] [Google Scholar]
- 117.Cassano GB, Heinze G, Lôo H, et al. A double-blind comparison of Tianeptine, Imipramine and placebo in the treatment of major depressive episodes. Eur Psychiatry 1996;11:254–9. 10.1016/0924-9338(96)82332-7 [DOI] [PubMed] [Google Scholar]
- 118.Dominguez RA, Goldstein BJ, Jacobson AF, et al. A double-blind placebo-controlled study of fluvoxamine and Imipramine in depression. J Clin Psychiatry 1985;46:84–7. [PubMed] [Google Scholar]
- 119.Merideth CH, Feighner JP. A double-blind, controlled evaluation of Zimeldine, Imipramine and placebo in patients with primary affective disorders. Acta Psychiatr Scand Suppl 1983;308:70–9. 10.1111/j.1600-0447.1983.tb11104.x [DOI] [PubMed] [Google Scholar]
- 120.Shrivastava RK, Shrivastava SH, Overweg N, et al. A double-blind comparison of paroxetine, Imipramine, and placebo in major depression. J Clin Psychiatry 1992;53 Suppl:48–51. Available: Suppl:48-51 [PubMed] [Google Scholar]
- 121.Cohn JB, Crowder JE, Wilcox CS, et al. A Placebo- and Imipramine-controlled study of paroxetine. Psychopharmacol Bull 1990;26:185–9. [PubMed] [Google Scholar]
- 122.Cohn JB, Wilcox CS. Paroxetine in major depression: a double-blind trial with Imipramine and placebo. J Clin Psychiatry 1992;53:52–6. [PubMed] [Google Scholar]
- 123.Feighner JP, Meredith CH, Frost NR, et al. A double-blind comparison of alprazolam vs. Imipramine and placebo in the treatment of major depressive disorder. Acta Psychiatr Scand 1983;68:223–33. 10.1111/j.1600-0447.1983.tb07003.x [DOI] [PubMed] [Google Scholar]
- 124.Feighner JP, Boyer WF, Merideth CH, et al. A double-blind comparison of fluoxetine, Imipramine and placebo in outpatients with major depression. Int Clin Psychopharmacol 1989;4:127–34. 10.1097/00004850-198904000-00004 [DOI] [PubMed] [Google Scholar]
- 125.Itil TM, Shrivastava RK, Mukherjee S, et al. A double-blind placebo-controlled study of fluvoxamine and Imipramine in out-patients with primary depression. Brit J Clinical Pharma 1983;15. 10.1111/j.1365-2125.1983.tb02134.x Available: https://bpspubs.onlinelibrary.wiley.com/toc/13652125/15/S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.March JS, Kobak KA, Jefferson JW, et al. A double-blind, placebo-controlled trial of fluvoxamine versus Imipramine in outpatients with major depression. J Clin Psychiatry 1990;51:200–2. [PubMed] [Google Scholar]
- 127.McGrath PJ, Stewart JW, Janal MN, et al. A placebo-controlled study of fluoxetine versus Imipramine in the acute treatment of atypical depression. Am J Psychiatry 2000;157:344–50. 10.1176/appi.ajp.157.3.344 [DOI] [PubMed] [Google Scholar]
- 128.Agosti V, McGrath PJ. Comparison of the effects of fluoxetine, Imipramine and placebo on personality in atypical depression. J Affect Disord 2002;71:113–20. 10.1016/s0165-0327(01)00393-7 [DOI] [PubMed] [Google Scholar]
- 129.Rickels K, Cohen D, Csanalosi I, et al. Alprazolam and Imipramine in depressed outpatients: A controlled study. Curr Ther Res Clin Exp 1982;32:157–64. [Google Scholar]
- 130.Roth D, Mattes J, Sheehan KH, et al. A double-blind comparison of fluvoxamine, desipramine and placebo in outpatients with depression. Prog Neuropsychopharmacol Biol Psychiatry 1990;14:929–39. 10.1016/0278-5846(90)90078-u [DOI] [PubMed] [Google Scholar]
- 131.Raisi F, Habibi N, Nasehi AA, et al. Combination of citalopram and nortriptyline in the treatment of severe major depression: a double-blind, placebo-controlled trial. Therapy 2007;4:187–92. 10.2217/14750708.4.2.187 [DOI] [Google Scholar]
- 132.Fabre LF. A 6-week, double-blind trial of paroxetine, Imipramine, and placebo in depressed outpatients. J Clin Psychiatry 1992;53 Suppl:40–3. Available: Suppl:40-3 [PubMed] [Google Scholar]
- 133.Feighner JP, Pambakian R, Fowler RC, et al. A comparison of Nefazodone, Imipramine, and placebo in patients with moderate to severe depression. Psychopharmacol Bull 1989;25:219–21. [PubMed] [Google Scholar]
- 134.Mann JJ, Georgotas A, Newton R, et al. A controlled study of trazodone, Imipramine, and placebo in outpatients with endogenous depression. J Clin Psychopharmacol 1981;1:75–80. 10.1097/00004714-198103000-00006 [DOI] [PubMed] [Google Scholar]
- 135.Schweizer E, Feighner J, Mandos LA, et al. Comparison of venlafaxine and Imipramine in the acute treatment of major depression in outpatients. J Clin Psychiatry 1994;55:104–8. [PubMed] [Google Scholar]
- 136.Escobar JI, Gomez J, Constain C, et al. Controlled clinical trial with trazodone, a novel antidepressant. A South American experience. J Clin Pharmacol 1980;20:124–30. 10.1002/j.1552-4604.1980.tb02534.x [DOI] [PubMed] [Google Scholar]
- 137.Ferguson JM, Mendels J, Manowitz NR. Dothiepin versus doxepin in major depression: results of a multicenter, placebo-controlled trial. J Clin Psychiatry 1994;55:258–63. [PubMed] [Google Scholar]
- 138.Bremner JD. Doppelblindvergleich von Mirtazapin, Amitriptylin und Plazebo BEI major depression. Nervenheilkunde 1996;15:533–40. [Google Scholar]
- 139.Feighner JP. A double-blind comparison of paroxetine, Imipramine and placebo in depressed outpatients. Int Clin Psychopharmacol 1992;6 Suppl 4:31–5. 10.1097/00004850-199206004-00007 [DOI] [PubMed] [Google Scholar]
- 140.Feighner JP, Cohn JB, Fabre LF, et al. A study comparing paroxetine placebo and Imipramine in depressed patients. J Affect Disord 1993;28:71–9. 10.1016/0165-0327(93)90035-i [DOI] [PubMed] [Google Scholar]
- 141.Feighner JP, Boyer WF. Paroxetine in the treatment of depression: a comparison with Imipramine and placebo. Acta Psychiatr Scand Suppl 1989;350:125–9. 10.1111/j.1600-0447.1989.tb07190.x [DOI] [PubMed] [Google Scholar]
- 142.Dunbar GC, Cohn JB, Fabre LF, et al. A comparison of paroxetine, Imipramine and placebo in depressed out-patients. Br J Psychiatry 1991;159:394–8. 10.1192/bjp.159.3.394 [DOI] [PubMed] [Google Scholar]
- 143.Stewart JW, Quitkin F, Liebowitz MR, et al. Efficacy of desipramine in mildly depressed patients: a double-blind, placebo-controlled trial. Psychopharmacol Bull 1981;17:136–8. [PubMed] [Google Scholar]
- 144.Emsley R, Ahokas A, Suarez A, et al. Efficacy of Tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week Placebo- and Escitalopram-controlled study. J Clin Psychiatry 2018;79:17m11741. 10.4088/JCP.17m11741 [DOI] [PubMed] [Google Scholar]
- 145.Van de Merwe TJ, Silverstone T, Ankier SI. Electrophysiological and Haemodynamic changes with trazodone, amitriptyline and placebo in depressed out-patients. Curr Med Res Opin 1984;9:339–52. 10.1185/03007998409109602 [DOI] [PubMed] [Google Scholar]
- 146.Gerner R, Estabrook W, Steuer J, et al. Treatment of geriatric depression with trazodone, Imipramine, and placebo: a double-blind study. J Clin Psychiatry 1980;41:216–20. [PubMed] [Google Scholar]
- 147.Gerner R, Estabrook W, Steuer J, et al. A placebo-controlled double-blind study of Imipramine and trazodone in geriatric depression. Proc Annu Meet Am Psychopathol Assoc 1980;69:167–82. [PubMed] [Google Scholar]
- 148.Hayes RL, Gerner RH, Fairbanks L, et al. ECG findings in geriatric Depressives given trazodone, placebo, or Imipramine. Journal of Clinical Psychopharmacology 1983;3:325. 10.1097/00004714-198310000-00017 [DOI] [PubMed] [Google Scholar]
- 149.Minelli A, Bortolomasi M, Scassellati C, et al. Effects of intravenous antidepressant drugs on the excitability of human motor cortex: a study with paired magnetic stimulation on depressed patients. Brain Stimul 2010;3:15–21. 10.1016/j.brs.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 150.Buchsbaum MS, Lee S, Haier R, et al. Effects of Amoxapine and Imipramine on evoked potentials in the continuous performance test in patients with affective disorder. Neuropsychobiology 1988;20:15–22. 10.1159/000118467 [DOI] [PubMed] [Google Scholar]
- 151.Klieser E, Lehmann E. Experimental examination of trazodone. Clin Neuropharmacol 1989;12 Suppl 1:S18–24. 10.1097/00002826-198901001-00004 [DOI] [PubMed] [Google Scholar]
- 152.Niklson IA, Reimitz PE, Sennef C. Factors that influence the outcome of placebo-controlled antidepressant clinical trials. Psychopharmacol Bull 1997;33:41–51. [PubMed] [Google Scholar]
- 153.Claghorn JL, Earl CQ, Walczak DD, et al. Fluvoxamine maleate in the treatment of depression: a single-center, double-blind, placebo-controlled comparison with Imipramine in outpatients. J Clin Psychopharmacol 1996;16:113–20. 10.1097/00004714-199604000-00003 [DOI] [PubMed] [Google Scholar]
- 154.Fabre L, Birkhimer LJ, Zaborny BA, et al. Fluvoxamine versus Imipramine and placebo: a double-blind comparison in depressed patients. Int Clin Psychopharmacol 1996;11:119–27. [PubMed] [Google Scholar]
- 155.Kocsis JH, Frances A, Mann JJ, et al. Imipramine for treatment of chronic depression. Psychopharmacol Bull 1985;21:698–700. [PubMed] [Google Scholar]
- 156.Kocsis JH, Frances AJ, Voss C, et al. Imipramine treatment for chronic depression. Arch Gen Psychiatry 1988;45:253. 10.1001/archpsyc.1988.01800270071008 [DOI] [PubMed] [Google Scholar]
- 157.Kocsis JH, Frances AJ, Voss C, et al. Imipramine and social-vocational adjustment in chronic depression. AJP 1988;145:997–9. 10.1176/ajp.145.8.997 [DOI] [PubMed] [Google Scholar]
- 158.Kocsis JH, Mason BJ, Frances AJ, et al. Prediction of response of chronic depression to Imipramine. Journal of Affective Disorders 1989;17:255–60. 10.1016/0165-0327(89)90008-6 [DOI] [PubMed] [Google Scholar]
- 159.Nair NP, Amin M, Holm P, et al. Moclobemide and nortriptyline in elderly depressed patients. A randomized, Multicentre trial against placebo. J Affect Disord 1995;33:1–9. 10.1016/0165-0327(94)00047-d [DOI] [PubMed] [Google Scholar]
- 160.Kin NM, Klitgaard N, Nair NP, et al. Clinical relevance of serum nortriptyline and 10-hydroxy-nortriptyline measurements in the depressed elderly: A multicenter pharmacokinetic and pharmacodynamic study. Neuropsychopharmacology 1996;15:1–6. 10.1016/0893-133X(95)00142-Z [DOI] [PubMed] [Google Scholar]
- 161.Ng Ying Kin NMK, Vasavan Nair NP, Amin M, et al. The dexamethasone suppression test and treatment outcome in elderly depressed patients participating in a placebo-controlled multicenter trial involving Moclobemide and nortriptyline. Biological Psychiatry 1997;42:925–31. 10.1016/S0006-3223(97)00158-3 [DOI] [PubMed] [Google Scholar]
- 162.Beasley CM, Sayler ME, Bosomworth JC, et al. High-dose fluoxetine: efficacy and activating-Sedating effects in agitated and retarded depression. J Clin Psychopharmacol 1991;11:166–74. [PubMed] [Google Scholar]
- 163.Stark P, Hardison CD. A review of multicenter controlled studies of fluoxetine vs. Imipramine and placebo in outpatients with major depressive disorder. J Clin Psychiatry 1985;46:53–8. [PubMed] [Google Scholar]
- 164.Cohn JB, Wilcox C. A comparison of fluoxetine, Imipramine, and placebo in patients with major depressive disorder. J Clin Psychiatry 1985;46:26–31. [PubMed] [Google Scholar]
- 165.Versiani M, Nardi AE, Mundim FD, et al. Moclobemide, Imipramine and placebo in the treatment of major depression. Acta Psychiatr Scand 1990;82:57–8. 10.1111/j.1600-0447.1990.tb05331.x Available: https://onlinelibrary.wiley.com/toc/16000447/82/S360 [DOI] [PubMed] [Google Scholar]
- 166.Versiani M, Nardi AE, Mundim FD, et al. Moclobemide, Imipramine, and placebo in the treatment of major depression (DSM III). J Neural Transm Suppl 1989;28:65–75. [PubMed] [Google Scholar]
- 167.Rickels K, Schweizer E, Clary C, et al. Nefazodone and Imipramine in major depression: a placebo-controlled trial. Br J Psychiatry 1994;164:802–5. 10.1192/bjp.164.6.802 [DOI] [PubMed] [Google Scholar]
- 168.Cohn CK, Robinson DS, Roberts DL, et al. Responders to antidepressant drug treatment: a study comparing Nefazodone, Imipramine, and placebo in patients with major depression. J Clin Psychiatry 1996;57 Suppl 2:15–8. [PubMed] [Google Scholar]
- 169.Miller HL, Ekstrom RD, Mason GA, et al. Noradrenergic function and clinical outcome in antidepressant Pharmacotherapy. Neuropsychopharmacology 2001;24:617–23. 10.1016/S0893-133X(00)00232-3 [DOI] [PubMed] [Google Scholar]
- 170.Rothblum ED, Sholomskas AJ, Berry C, et al. Issues in clinical trials with the depressed elderly. J Am Geriatr Soc 1982;30:694–9. 10.1111/j.1532-5415.1982.tb01982.x [DOI] [PubMed] [Google Scholar]
- 171.Weissman MM, Prusoff B, Sholomskas AJ, et al. A double-blind clinical trial of alprazolam, Imipramine, or placebo in the depressed elderly. J Clin Psychopharmacol 1992;12:175–82. [PubMed] [Google Scholar]
- 172.Weissman MM, Prusoff BA, Sholomskas AJ, et al. The pharmacologic treatment of the depressed elderly: A pilot study of alprazolam (Xanax), Imipramine (Tofranil) or placebo. In: Burrows GD, Norman TR, Maguire KP, eds. Biological psychiatry: recent studies. London: John Libbey, 1984: 167–74. [Google Scholar]
- 173.Rocha FL, Soares JF. Multicentre, double-blind, placebo controlled study of Tianeptine in major depressive episodes. traditional statistical analysis and the unbalanced repeated measures models analysis. J Bras Psiquiatr 1998;47:105–17. [Google Scholar]
- 174.Costa e Silva JA, Ruschel SI, Caetano D, et al. Placebo-controlled study of Tianeptine in major depressive episodes. Neuropsychobiology 1997;35:24–9. 10.1159/000119326 [DOI] [PubMed] [Google Scholar]
- 175.Montenegro R. Eficacia de la Tianeptina en El Tratamiento de Los Episodios de Depresión Mayor con O sin Melancolía. Estudio en doble Ciego: Tianeptina versus placebo. Arq Bras Med 1997;71:193–4. [Google Scholar]
- 176.Katz MM, Houston JP, Brannan S, et al. A Video method for the evaluation of antidepressant clinical and behavioural actions. Int J Neuropsychopharmacol 2006;9:327–36. 10.1017/S1461145705005778 [DOI] [PubMed] [Google Scholar]
- 177.Katz MM, Tekell JL, Bowden CL, et al. Onset and early behavioral effects of Pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology 2004;29:566–79. 10.1038/sj.npp.1300341 [DOI] [PubMed] [Google Scholar]
- 178.Javors MA, Houston JP, Tekell JL, et al. Reduction of platelet serotonin content in depressed patients treated with either paroxetine or desipramine. Int J Neuropsychopharm 2000;3:229–35. 10.1017/S146114570000198X [DOI] [PubMed] [Google Scholar]
- 179.Pomara N, Shao B, Choi SJ, et al. Sex-related differences in nortriptyline-induced side-effects among depressed patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2001;25:1035–48. 10.1016/S0278-5846(01)00175-0 [DOI] [PubMed] [Google Scholar]
- 180.Edwards JG, Goldie A. Mianserin, Maprotiline and Intracardiac conduction. Brit J Clinical Pharma 1983;15. 10.1111/j.1365-2125.1983.tb05872.x Available: https://bpspubs.onlinelibrary.wiley.com/toc/13652125/15/S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Edwards JG, Goldie A. Placebo-controlled trial of Mianserin and Maprotiline in primary depressive illness: a preliminary report. Br J Clin Pharmacol 1983;15:239S–248S. 10.1111/j.1365-2125.1983.tb05871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sedgwick EM, Edwards JG. Mianserin, Maprotiline and the electroencephalogram. Br J Clin Pharmacol 1983;1:255S–259S. 10.1111/j.1365-2125.1983.tb05873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stewart JW, Garfinkel R, Nunes EV, et al. Atypical features and treatment response in the National Institute of mental health treatment of depression collaborative research program. J Clin Psychopharmacol 1998;18:429–34. 10.1097/00004714-199812000-00002 [DOI] [PubMed] [Google Scholar]
- 184.Shea MT, Elkin I, Imber SD, et al. Course of depressive symptoms over follow-up. Arch Gen Psychiatry 1992;49:782. 10.1001/archpsyc.1992.01820100026006 [DOI] [PubMed] [Google Scholar]
- 185.Elkin I, Gibbons RD, Shea MT, et al. Initial severity and differential treatment outcome in the National Institute of mental health treatment of depression collaborative research program. J Consult Clin Psychol 1995;63:841–7. 10.1037//0022-006x.63.5.841 [DOI] [PubMed] [Google Scholar]
- 186.Elkin I, Parloff MB, Hadley SW, et al. NIMH treatment of depression collaborative research program. background and research plan. Arch Gen Psychiatry 1985;42:305–16. 10.1001/archpsyc.1985.01790260103013 [DOI] [PubMed] [Google Scholar]
- 187.Elkin I, Shea MT, Watkins JT, et al. National Institute of mental health treatment of depression collaborative research program, General effectiveness of treatments. Arch Gen Psychiatry 1989;46:971–82. 10.1001/archpsyc.1989.01810110013002 [DOI] [PubMed] [Google Scholar]
- 188.Sotsky SM, Glass DR, Shea MT, et al. Patient predictors of response to psychotherapy and Pharmacotherapy: findings in the NIMH treatment of depression collaborative research program. Am J Psychiatry 1991;148:997–1008. 10.1176/ajp.148.8.997 [DOI] [PubMed] [Google Scholar]
- 189.Sotsky SM, Simmens SJ. Pharmacotherapy response and diagnostic validity in atypical depression. J Affect Disord 1999;54:237–47. 10.1016/s0165-0327(99)00014-2 [DOI] [PubMed] [Google Scholar]
- 190.Krupnick JL, Elkin I, Collins J, et al. Therapeutic alliance and clinical outcome in the NIMH treatment of depression collaborative research program: preliminary findings. Psychotherapy: Theory, Research, Practice, Training 1994;31:28–35. 10.1037/0033-3204.31.1.28 [DOI] [Google Scholar]
- 191.Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and Pharmacotherapy outcome: findings in the National Institute of mental health treatment of depression collaborative research program. J Consult Clin Psychol 1996;64:532–9. 10.1037//0022-006x.64.3.532 [DOI] [PubMed] [Google Scholar]
- 192.Collins JF, Elkin I. Randomization in the NIMH treatment of depression collaborative research program. New Directions for Program Evaluation 1985;1985:27–37. 10.1002/ev.1407 Available: https://onlinelibrary.wiley.com/toc/15512371/1985/28 [DOI] [Google Scholar]
- 193.Imber SD, Pilkonis PA, Sotsky SM, et al. Mode-specific effects among three treatments for depression. J Consult Clin Psychol 1990;58:352–9. 10.1037//0022-006x.58.3.352 [DOI] [PubMed] [Google Scholar]
- 194.Watkins JT, Leber WR, Imber SD, et al. Temporal course of change of depression. J Consult Clin Psychol 1993;61:858–64. 10.1037//0022-006x.61.5.858 [DOI] [PubMed] [Google Scholar]
- 195.Katz I. Steady state pharmacokinetics of nortriptyline in the frail elderly. Neuropsychopharmacology 1989;2:229–36. 10.1016/0893-133X(89)90026-2 [DOI] [PubMed] [Google Scholar]
- 196.Katz IR, Simpson GM, Curlik SM, et al. Pharmacologic treatment of major depression for elderly patients in residential care settings. J Clin Psychiatry 1990;51 Suppl:41–7. [PubMed] [Google Scholar]
- 197.Murphy GE, Simons AD, Wetzel RD. Plasma nortriptyline and clinical response in depression. Journal of Affective Disorders 1985;8:123–9. 10.1016/0165-0327(85)90034-5 [DOI] [PubMed] [Google Scholar]
- 198.Murphy GE, Simons AD, Wetzel RD, et al. Cognitive therapy and Pharmacotherapy. singly and together in the treatment of depression. Arch Gen Psychiatry 1984;41:33–41. 10.1001/archpsyc.1984.01790120037006 [DOI] [PubMed] [Google Scholar]
- 199.Simons AD, Levine JL, Lustman PJ, et al. Patient attrition in a comparative outcome study of depression. Journal of Affective Disorders 1984;6:163–73. 10.1016/0165-0327(84)90021-1 [DOI] [PubMed] [Google Scholar]
- 200.Simons AD, Murphy GE, Levine JL, et al. Cognitive therapy and Pharmacotherapy for depression. sustained improvement over one year. Arch Gen Psychiatry 1986;43:43–8. 10.1001/archpsyc.1986.01800010045006 [DOI] [PubMed] [Google Scholar]
- 201.Ginestet D. Efficacy of Tianeptine in major depressive disorders with or without Melancholia. Eur Neuropsychopharmacol 1997;7:S341–5. 10.1016/s0924-977x(97)00067-9 [DOI] [PubMed] [Google Scholar]
- 202.Amin MM, Ananth JV, Coleman BS, et al. Fluvoxamine: antidepressant effects confirmed in a placebo-controlled international study. Clin Neuropharmacol 1984;7:317. 10.1097/00002826-198406001-00286 [DOI] [Google Scholar]
- 203.Cassano GB, Conti L, Massimetti G, et al. Use of a standardized documentation system (BLIPS/BDP) in the conduct of a multicenter International trial comparing fluvoxamine, Imipramine, and placebo. Psychopharmacol Bull 1986;22:52–8. [PubMed] [Google Scholar]
- 204.Ottevanger EA. The efficacy of fluvoxamine in patients with severe depression. Prog Neuropsychopharmacol Biol Psychiatry 1994;18:731–40. 10.1016/0278-5846(94)90080-9 [DOI] [PubMed] [Google Scholar]
- 205.Ottevanger EA. Efficacite de la fluvoxamine Chez des patients Atteints de depression severe. Psychol Med 1991;23:1639–44. [Google Scholar]
- 206.Wagner W, Cimander K, Schnitkerb J, et al. Influence of concomitant psychotropic medication on the efficacy and tolerance of fluvoxamine. Adv Pharmacother 1986;2:34–56. [Google Scholar]
- 207.Ravindran AV, Teehan MD, Bakish D, et al. The impact of sertraline, desipramine, and placebo on psychomotor functioning in depression. Human Psychopharmacology 1995;10:273–81. 10.1002/hup.470100404 Available: https://onlinelibrary.wiley.com/toc/10991077/10/4 [DOI] [Google Scholar]
- 208.Peselow ED, Stanley M, Filippi AM, et al. The predictive value of the dexamethasone suppression test. A placebo-controlled study. Br J Psychiatry 1989;155:667–72. 10.1192/s0007125000018171 [DOI] [PubMed] [Google Scholar]
- 209.White K, Razani J, Cadow B, et al. Tranylcypromine vs nortriptyline vs placebo in depressed outpatients: a controlled trial. Psychopharmacology 1984;82:258–62. 10.1007/BF00427786 [DOI] [PubMed] [Google Scholar]
- 210.Feighner JP. Trazodone, a Triazolopyridine derivative, in primary depressive disorder. J Clin Psychiatry 1980;41:250–5. [PubMed] [Google Scholar]
- 211.Reynolds CF, Miller MD, Pasternak RE, et al. Treatment of bereavement-related major depressive episodes in later life: A controlled study of acute and continuation treatment with nortriptyline and Interpersonal psychotherapy. Am J Psychiatry 1999;156:202–8. 10.1176/ajp.156.2.202 [DOI] [PubMed] [Google Scholar]
- 212.Gelenberg AJ, Wojcik JD, Falk WE, et al. Tyrosine for depression: a double-blind trial. J Affect Disord 1990;19:125–32. 10.1016/0165-0327(90)90017-3 [DOI] [PubMed] [Google Scholar]
- 213.Cohn JB, Varga L, Lyford A. A two-center double-blind study of nomifensine, Imipramine, and placebo in depressed geriatric outpatients. J Clin Psychiatry 1984;45:68–72. [PubMed] [Google Scholar]
- 214.Merideth CH, Feighner JP, Hendrickson G. A double-blind comparative evaluation of the efficacy and safety of nomifensine, Imipramine, and placebo in depressed geriatric outpatients. J Clin Psychiatry 1984;45:73–7. [PubMed] [Google Scholar]
- 215.Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula Angustifolia mill. tincture and Imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:123–7. 10.1016/s0278-5846(02)00342-1 [DOI] [PubMed] [Google Scholar]
- 216.Gershon S, Mann J, Newton R, et al. Evaluation of trazodone in the treatment of endogenous depression: results of a multicenter double-blind study. J Clin Psychopharmacol 1981;1:39S–44S. 10.1097/00004714-198111001-00008 [DOI] [PubMed] [Google Scholar]
- 217.Gershon S, Newton R. Lack of anticholinergic side effects with a new Antidepressent--trazodone. J Clin Psychiatry 1980;41:100–4. [PubMed] [Google Scholar]
- 218.Lapierre YD, Browne M, Horn E, et al. Treatment of major affective disorder with fluvoxamine. J Clin Psychiatry 1987;48:65–8. [PubMed] [Google Scholar]
- 219.Wichers MC, Barge-Schaapveld DQCM, Nicolson NA, et al. Reduced stress-sensitivity or increased reward experience: the psychological mechanism of response to antidepressant medication. Neuropsychopharmacology 2009;34:923–31. 10.1038/npp.2008.66 [DOI] [PubMed] [Google Scholar]
- 220.Institut de Recherches Internationales Servier . Clinical study report Synopsis. 2021.
- 221.Bech P. Meta-analysis of placebo-controlled trials with Mirtazapine using the core items of the Hamilton depression scale as evidence of a pure antidepressive effect in the short-term treatment of major depression. Int J Neuropsychopharmacol 2001;4:337–45. 10.1017/S1461145701002565 [DOI] [PubMed] [Google Scholar]
- 222.Bech P. Comparison of ORG-3770 and amitriptyline in depressed outpatients. 1990. in: Bech, P. meta-analysis of placebo-controlled trials with Mirtazapine using the core items of the Hamilton depression scale as evidence of a pure antidepressive effect in the short-term treatment of major depression. Int J Neuropsychopharmacol 2001;4:337–45. 10.1017/S1461145701002565 [DOI] [PubMed] [Google Scholar]
- 223.Rickels K, Weise CC, Sandler K, et al. Nomifensine, Imipramine and placebo in depressed outpatients. Int Pharmacopsychiatry 1982;17 Suppl 1:73–88. 10.1159/000468602 [DOI] [PubMed] [Google Scholar]
- 224.Rickels K, Weise CC, Zal HM, et al. Lofepramine and Imipramine in Unipolar depressed outpatients. A placebo controlled study. Acta Psychiatr Scand 1982;66:109–20. 10.1111/j.1600-0447.1982.tb00919.x Available: https://onlinelibrary.wiley.com/toc/16000447/66/2 [DOI] [PubMed] [Google Scholar]
- 225.Kusalic M, Engelsmann F, Bradwejn J. Thyroid functioning during treatment for depression. J Psychiatry Neurosci 1993;18:260–3. [PMC free article] [PubMed] [Google Scholar]
- 226.Hormazabal L, Omer LM, Ismail S. Cianopramine and amitriptyline in the treatment of depressed patients--a placebo-controlled study. Psychopharmacology (Berl) 1985;86:205–8. 10.1007/BF00431710 [DOI] [PubMed] [Google Scholar]
- 227.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for systematic reviews of interventions: Cochrane. 2021. Available: www.training.cochrane.org/handbook
- 228.Golder S, Loke YK, Wright K, et al. Reporting of adverse events in published and unpublished studies of health care interventions: A systematic review. PLoS Med 2016;13:e1002127. 10.1371/journal.pmed.1002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tang E, Ravaud P, Riveros C, et al. Comparison of serious adverse events posted at Clinicaltrials.Gov and published in corresponding Journal articles. BMC Med 2015;13:189. 10.1186/s12916-015-0430-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Maund E, Tendal B, Hróbjartsson A, et al. Benefits and harms in clinical trials of Duloxetine for treatment of major depressive disorder: comparison of clinical study reports, trial registries, and publications. BMJ 2014;348:g3510. 10.1136/bmj.g3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Johnson CF, Macdonald HJ, Atkinson P, et al. Reviewing long-term antidepressants can reduce drug burden: a prospective observational cohort study. Br J Gen Pract 2012;62:e773–9. 10.3399/bjgp12X658304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. J Clin Psychiatry 2014;75:169–77. 10.4088/JCP.13m08443 [DOI] [PubMed] [Google Scholar]
- 233.Kinrys G, Gold AK, Pisano VD, et al. Tachyphylaxis in major depressive disorder: A review of the current state of research. J Affect Disord 2019;245:488–97. 10.1016/j.jad.2018.10.357 [DOI] [PubMed] [Google Scholar]
- 234.Gluud LL. Bias in clinical intervention research. Am J Epidemiol 2006;163:493–501. 10.1093/aje/kwj069 [DOI] [PubMed] [Google Scholar]
- 235.Kjaergard LL, Villumsen J, Gluud C. Reported Methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. 10.7326/0003-4819-135-11-200112040-00010 [DOI] [PubMed] [Google Scholar]
- 236.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–12. 10.1001/jama.273.5.408 [DOI] [PubMed] [Google Scholar]
- 237.Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, et al. Bias due to lack of patient blinding in clinical trials. A systematic review of trials Randomizing patients to blind and Nonblind sub-studies. Int J Epidemiol 2014;43:1272–83. 10.1093/ije/dyu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hróbjartsson A, Thomsen ASS, Emanuelsson F, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and Nonblinded assessors. CMAJ 2013;185:E201–11. 10.1503/cmaj.120744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hróbjartsson A, Thomsen ASS, Emanuelsson F, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012;344:bmj.e1119. 10.1136/bmj.e1119 [DOI] [PubMed] [Google Scholar]
- 240.Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42–6. 10.1136/bmj.323.7303.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses. Lancet 1998;352:609–13. 10.1016/S0140-6736(98)01085-X [DOI] [PubMed] [Google Scholar]
- 242.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjment-2023-300730supp001.pdf (4.9MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.