Abstract
Introduction
Acute brain injury can lead to states of decreased consciousness, that is, disorder of consciousness (DoC). Detecting signs of consciousness early is vital for DoC management in the intensive care unit (ICU), neurorehabilitation and long-term prognosis. Our primary objective is to investigate the potential of pharmacological stimulant therapies in eliciting signs of consciousness among unresponsive or low-responsive acute DoC patients.
Methods
In a placebo-controlled, randomised, cross-over setting, we evaluate the effect of methylphenidate and apomorphine in 50 DoC patients with acute traumatic or non-traumatic brain injury admitted to the ICU. Patients are examined before and after administration of the trial drugs using (1) neurobehavioural scales to determine the clinical level of consciousness, (2) automated pupillometry to record pupillary responses as a signature for awareness and (3) near-infrared spectroscopy combined with electroencephalography to record neurovascular coupling as a measure for cortical activity. Primary outcomes include pupillary dilations and increase in cortical activity during passive and active paradigms.
Ethics
The study has been approved by the ethics committee (Journal-nr: H-21022096) and follows the principles of the Declaration of Helsinki. It is deemed to pose minimal risks and to hold a significant potential to improve treatment options for DoC patients. If the stimulants are shown to enhance cortical modulation of pupillary function and neurovascular coupling, this would warrant a large multicentre trial to evaluate their clinical impact.
Dissemination
Results will be available on EudraCT, clinicaltrialsregister.eu and published in an international peer-reviewed journal.
Trial registration number
EudraCT Number: 2021-001453-31.
Keywords: CONSCIOUSNESS, CLINICAL NEUROLOGY, TRAUMATIC BRAIN INJURY, COMA, INTENSIVE CARE
WHAT IS ALREADY KNOWN ON THIS TOPIC
This first study to investigate multiple treatments in patients with acute disorders of consciousness aims at promoting consciousness.
WHAT THIS STUDY ADDS
It focuses on identifying novel biological markers of consciousness through bedside technologies, including automated pupillometry and near-infrared spectroscopy combined with electroencephalography.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The goal is to determine if apomorphine and methylphenidate can effectively enhance cortical modulation and neurovascular coupling, providing a basis for more extensive research into their clinical utility in the intensive care unit setting.
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study is the first to investigate multiple consciousness-promoting treatments in acute disorders of consciousness (DoC) patients in the intensive care unit (ICU).
The cross-over design helps to test different treatments in a relatively small sample size of difficult-to-enrol ICU patients.
Nevertheless, compared with other interventional DoC studies, our sample size is comparatively large.
Multimodal assessments including clinical examination, automated pupillometry and near-infrared spectroscopy combined with electroencephalography (NIRS-EEG) allows to investigate both clinical and proxy consciousness biomarkers.
Extraventricular drains and other intracranial devices may interfere with the placement of an NIRS-EEG cap, leading to potential selection bias of enrolled patients.
Introduction
Searching for signs of preserved consciousness in clinically unresponsive patients with brain injury
Patients with severe acute brain injury typically enter a coma.1 While many either die2 3 or quickly recover consciousness, others remain within the broad spectrum of disorders of consciousness (DoC) for weeks, months or even years.4–8 It is crucial for DoC patients in the intensive care unit (ICU) that signs of (residual) consciousness can be detected as early as possible,9–13 as the recovery of consciousness is the most critical prognostic factor for long-term outcome.14 15 In fact, when residual consciousness is overlooked, which may happen in up to 40% of DoC patients,16 this can have severe consequences because 70% of deaths in the ICU occur following a decision to withdraw life-sustaining therapy.17 For patients with severe acute brain injury, these decisions hinge on expectations regarding the patient’s potential to recover consciousness, even though the accuracy of current prognostic indicators for assessing this potential is limited.18
Perhaps even more concerning is that 15%–20% of clinically unresponsive DoC patients exhibit signs of preserved consciousness when advanced electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) technologies are employed.19–22 Unfortunately, these patients go undetected in daily ICU practice because such technologies are not clinical routine.23
We have previously found neurovascular coupling between functional near-infrared spectroscopy (fNIRS) oxyhaemoglobin (0.07–0.13 Hz) and EEG band-power (1–12 Hz) signals based on wavelet coherence at the frontal areas to be sensitive and prognostic to changing consciousness levels.24 Furthermore, we have shown the possibility of detecting covert consciousness in patients with brain injuries when using automated pupillometry combined with active and passive paradigms.25 Applying these tools in the setting of ICU could provide us with more clinical information and help detect patients with preserved consciousness.
Pharmacological stimulant therapy for clinically unresponsive patients with brain injury
Pharmacological stimulant therapies such as apomorphine and methylphenidate can be administered in chronic DoC to stimulate arousal and awareness with low risks of serious adverse effects.26–32 Apomorphine is a potent dopamine agonist with direct stimulating effects on D1 receptors and D2 receptors. It is indicated for Parkinson’s patients with motor on-off phenomenon when treatment with levodopa is not sufficient any longer.33 In case reports and small-scale studies involving patients in a subacute to chronic state of DoC following traumatic brain injury (TBI), improvement in neurobehavioural scales was observed within days to weeks after daily infusion of ≥2 mg subcutaneous apomorphine.34 35 Methylphenidate is a sympathomimetic stimulant with a more prominent effect on mental compared with motor activity. Methylphenidate increases the synaptic concentrations of dopamine and norepinephrine by inhibiting the reuptake in the striatum.36 Postcardiac arrest patients treated with either methylphenidate or amantadine showed improvements in terms of command following, survival until discharge and modified Rankin scale scores.37 Similarly, TBI patients treated with 0.3 mg/kg methylphenidate two times per day had significantly shorter ICU admissions, and in cases of severe injuries, it also resulted in reduced hospital admissions.38 Although the clinical effect size seems modest, stimulants may thus improve functional and cognitive function in patients with other chronic brain injuries.39–41
Stimulants to detect signatures of consciousness recovery before clinical improvement
Neither apomorphine nor methylphenidate have been firmly evaluated in the acute phase of traumatic or non-TBIs. Given the modest clinical effect size, very large trials would probably be necessary to detect effects on clinical outcomes. In the ICU, this is not feasible with the current state of evidence. To bridge this gap, we suggest that a smaller trial could show if stimulants can improve biological signatures of preserved consciousness, using easy-to-implement bedside technologies, before detectable clinical improvement occurs. In other words, if stimulants such as apomorphine or methylphenidate could improve cortical modulation of pupillary function (detectable with automated pupillometry,25 42) and/or neurovascular coupling in the brain (detectable with NIRS-EEG,24), then larger trials might be warranted to assess clinical effects.
Study objectives
The primary objective is to investigate, in a placebo-controlled, randomised, cross-over setting: (1) the potential effects on pupillary function and neurovascular coupling with administrations of 20 mg methylphenidate in patients with acute DoC and (2) the potential effects on pupillary function and neurovascular coupling with subcutaneous injections of 2 mg apomorphine in patients with acute DoC (proxy biomarkers for consciousness levels).
The secondary objective is to assess, in a placebo-controlled setting, potential clinical effects on consciousness with administration of 20 mg methylphenidate and subcutaneous injection of 2 mg apomorphine, respectively, in patients with acute DoC (clinical consciousness levels).
Methods and analysis
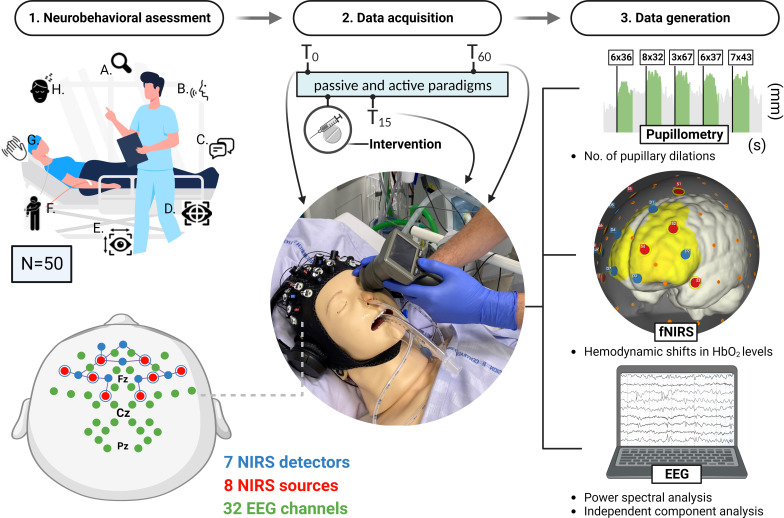
We will include 50 patients with DoC and acute traumatic or non-TBI. Each patient is examined at inclusion using the Full Outline of UnResponsiveness (FOUR43) and Simplified Evaluation of CONsciousness Disorders (SECONDs44) scales to determine the suitable diagnosis of DoC: coma, vegetative state (VS)/unresponsive wakefulness syndrome (UWS), minimally conscious state (MCS) (minus/plus) or emergence from MCS (see table 1 for definitions and references). Patients meeting the inclusion criteria undergo a baseline assessment (T0) that includes automated pupillometry measurements and NIRS-EEG combined with passive and active paradigms before receiving the randomised drug. Subsequently, repeated assessments are conducted at 15 min (T15) and 60 min (T60) after drug administration to align with the peak plasma concentration of apomorphine and methylphenidate, respectively. Figure 1 illustrates the clinical protocol overview.
Table 1.
Disorder of consciousness
| Disorder of consciousness | Definition |
| Coma67 68 | A state of profound unawareness, unresponsive to arousal, with an absent normal sleep-wake cycle. Typically lasts a few days to 3 weeks postacute brain injury. |
| Vegetative state/unresponsive wakefulness syndrome (VS/UWS)69 | A state of wakefulness without awareness, where patients may open their eyes but exhibit only reflex behaviours. |
| Minimally conscious state (MCS)4 5 | A state where the patients may exhibit inconsistent yet reproducible non-reflex behaviours in response to environmental stimuli. Patients are classified as MCS if they show signs such as pain localisation, visual fixation/tracking, appropriate emotional expressions (MCS minus), or if they can follow commands (MCS plus). |
| Emergence from MCS (eMCS)70 | A state characterised by the recovery of functional communication (eg, the ability to answer yes/no questions) and/or the use of objects (correctly using at least two different everyday objects). |
Figure 1.
Procedural overview of neurobehavioural assessment and cognitive function using Simplified Evaluation of CONsciousness Disorders (SECONDs) scale with bedside automated pupillometry and near-infrared spectroscopy combined with electroencephalograph (NIRS-EEG). Neurobehavioural evaluation using the SECONDs scale. This evaluation encompasses eight items: (A) observation of the patient’s spontaneous behaviour, (B) the patient’s ability to follow commands, (C) the capacity for communication, (D) the ability to visually track, (E) the ability to visually track fixate, (F) localisation of pain, (G) the display of oriented behaviour and (H) the degree of arousal, particularly eye-opening responsiveness. The patient is eligible for inclusion if items B and C are clinically absent. The data acquisition phase involves using automated pupillometry and an NIRS-EEG set-up—integrating near-infrared spectroscopy and EEG—to monitor patients’ responses to auditory and mental arithmetic stimuli. This multimodal approach records pupillary responses, haemodynamic changes in brain oxygenation (HbO2 levels) and EEG brain activity. The set-up for the NIRS-EEG includes seven detectors, eight sources with short channels and a comprehensive 32-channel EEG. Data recording occurs three times within each visit: initially at baseline (T0), then following a pharmacological intervention at 15 min (T15), and lastly after 60 min (T60). From the data collection process, three key types of data are generated. First, the protocol measures the frequency of pupillary dilations for each stimulus paradigm, which reflects the degree of induced cognitive load. Second, it monitors changes in haemodynamic HbO2 levels in the brain, providing insights into cerebral blood flow and oxygenation. Third, it examines EEG activity, interpretated by power spectral analysis and independent component analysis. The interplay between NIRS HbO2 concentrations and EEG band-power will finally be analysed to assess a potential increase in neurovascular coupling following drug intervention. The figure is an original work created by the first author (MHO), who has granted permission for its reuse in this context.
Study recruitment and setting
Patients with acute DoC due to acute brain injury are prospectively recruited from the neurocritical, cardiological, cardiothoracic and general ICUs of a tertiary referral centre (Rigshospitalet, Copenhagen, Denmark) plus a general ICU of another site (Bispebjerg Hospital, Copenhagen, Denmark). Screening and identification of participants are carried out by primary investigator MHO.
Inclusion of study population
Age ≥18 years.
Patients with severe acute traumatic or non-TBI in an unresponsive state (coma, vs/UWS) or a low-responsive state (MCS) according to FOUR and SECONDs.
Written informed consent for trial participation from next-of-kin.
Exclusion of study population
Recovery of the ability to follow commands prior to enrolment.
Pre-existing DoC.
Pre-existing mental or severe physical impairments.
Deafness or eye disease interfering with pupillary evaluations.
Use of dopamine agonists or antagonists within six half-lives of the drug.
Use of psychoactive or psychotropic substances within six half-lives of the drug.
Clinically unstable requiring immediate neurological, medical or surgical management.
Consent
Once a patient is identified as a potential participant, the primary investigator proceeds to inform the next-of-kin about the clinical trial. If the latter is interested, a meeting is planned in a confidential environment to deliver relevant information regarding the study. Provided a written consent is obtained, a trial guardian is contacted to ensure that the decision to participate in the study is made in accordance with the study criteria.
Intervention
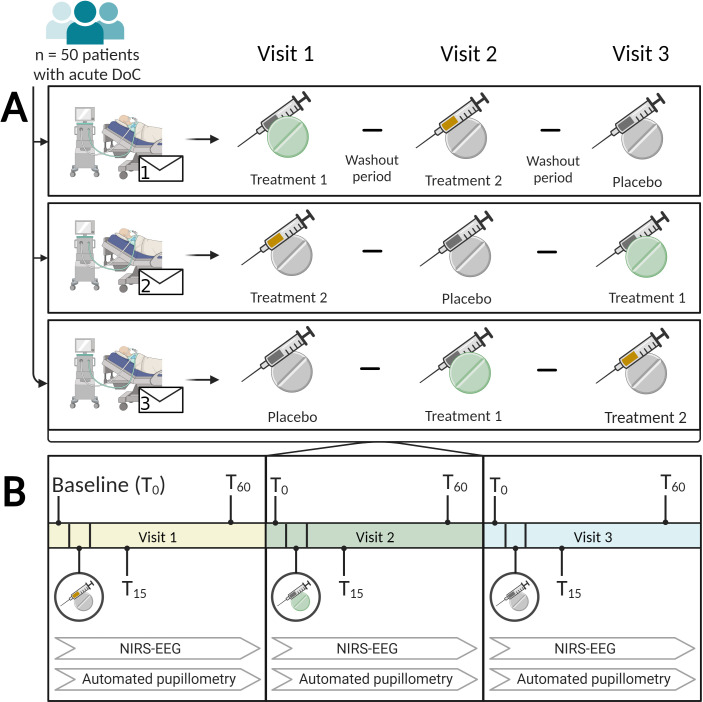
Patients are randomised at inclusion to a schedule that consists of three consecutive visits, table 2 demonstrates the stratified randomisation. Each visit contains an administration of one of three arms (two of which containing the active drug). The patients can have one or two visits per day with a minimum of 10 hours wash-out period in between to minimise the risk of carry-over effects. By completing a baseline followed by two assessments, patients will serve as their own controls, thereby reducing the risk of natural recovery as a time-modified confounder.45
Table 2.
Randomisation and treatment order
| Treatment order | Visit 1 | Visit 2 | Visit 3 |
| 1 | Apomorphine | Methylphenidate | Placebo |
| 2 | Apomorphine | Placebo | Methylphenidate |
| 3 | Methylphenidate | Apomorphine | Placebo |
| 4 | Methylphenidate | Placebo | Apomorphine |
| 5 | Placebo | Apomorphine | Methylphenidate |
| 6 | Placebo | Methylphenidate | Apomorphine |
In each visit, the treatment will comprise either (1) 20 mg methylphenidate tablet suspended in water and administered through a nasogastric (NG) tube, (2) 2 mg subcutaneous injection of apomorphine or (3) saline as placebo, administered through an NG tube or a subcutaneous injection.
To prevent observer bias and to examine effects of the active drugs in the best possible way, we are administering subcutaneous placebo injections matched for apomorphine fluid volume or placebo tablets suspended in water matched for methylphenidate fluid volume alongside each given drug at the particular session, for example, if the primary drug is to be an injection, a placebo tablet is suspended in water and administered through an NG tube and vice versa. See figure 2 for illustration of randomisation approach and protocol procedure.
Figure 2.
Randomisation approach and administration methods for treatments (A). Fifty patients with acute disorders of consciousness (either vegetative state/unresponsive wakefulness syndrome or minimal consciousness state) following acute brain injury are assigned randomly to a schedule of three-arm treatments spread over three visits. These treatments consist of 2 mg apomorphine (orange), 20 mg methylphenidate (green) and a placebo (grey). The clinical assessment process for each visit (B). During each visit, the patients undergo three examinations incorporating automated pupillometry and near-infrared spectroscopy combined with electroencephalography (NIRS-EEG), along with passive and active paradigms. Each examination follows a timeline. First, a baseline assessment (T0), which is followed by administration of the first drug. Subsequently, the examination is repeated at two time points: 15 min (T15) and 60 min (T60) after administration of the drug. The second and third visits follow the same pattern, but with administration of the second and third drugs, respectively. The figure is an original work created using biorender.com by the first and the last authors (MHO, DK), who have granted permission for its reuse in this context. DoC, disorder of consciousness.
Automated pupillometry
Pupillary responses to passive and active paradigms are recorded using the NeurOptics PLR-3000 pupillometer, which we have previously used in the context of acute DoC.25 The objective is to trace signs of consciousness through active command-following tasks and passive auditory stimulation measured by pupillary dilation as a cortically mediated response.
Near-infrared spectroscopy combined with electroencephalography
Recording neurovascular coupling in acute DoC is feasible and can effectively distinguish between levels of consciousness when processed with a machine learning algorithm.24 In this context, the device is used to assess the neurovascular coupling in acute DoC following stimulant therapy, as outlined above.
Establishment of research biobank
To elucidate the pharmacokinetic-pharmacodynamic relationship of the administered drugs, blood samples of approximately 8 mL will be drawn at two specific time points, T15 and T60. These time points aim to capture the drug’s absorption and potential peak plasma concentration. Due to the rapid autooxidation of apomorphine, we will collect blood samples using prechilled vacutainers containing K2EDTA and sodium fluoride as anticoagulants. Immediately after the blood draw, we will add a 0.15 mL solution of 6% ascorbic acid and mix it thoroughly.46 47 The samples will then be centrifuged and securely stored at −80°C in a research biobank. These samples will be analysed to determine the plasma concentration of the administered dosages. By doing so, we can correlate the observed clinical effects, pupillary responses and increased neurovascular coupling with the actual drug levels in the bloodstream, offering insights into how the trial drugs exert their effects.
Preparation of drugs
Preparation of trials drugs is handled by research assistant nurses, while injections and tablets are given by the blinded investigator. The research assistant nurses will have at least one coobserver, and both are obliged to sign and fill out a medical form to verify the allocated treatment according to the randomisation. This form will include the patient id, name of the given drug, date and time. The trial drugs have been approved by the Danish Medicine Agency and maximum tolerated dose is used to ensure that no positive effects are overlooked.
Unblinding procedure
To accommodate any emergency unblinding, a 24-hour emergency number to investigator is available in the ward and the electronic health records. Moreover, a sealed emergency unblinding envelope revealing the allocated intervention is placed next to the trial participants for at least the amount of time it takes for the drugs to be considered ‘washed-out’.
Power and sample size calculations
For this placebo-controlled, randomised, cross-over study, the sample size was determined using G*Power V.3.1 software.48 49 Separate sample sizes were calculated for each primary outcome, and the largest sample size was selected.
Preliminary data from automated pupillometry combined with mental tasks revealed success rates of 70% (14 out of 20) among healthy volunteers and 39.5% (17 out of 43) among neurological patients.25 To detect a clinically relevant difference in pupillary dilations between baseline and after drug administration with a power of 0.80 and a two-tailed alpha of 0.05, we calculated a required control and case group size each of 41 subjects.
A similar calculation was conducted using the Wilcoxon-Mann-Whitney-U test to determine the required sample size for detecting a difference in neurovascular coupling. This calculation was based on preliminary NIRS-EEG data from two groups: ICU patients (n=9) and control neurological patients (n=14).24 An effect size of 1.07 was computed (means, 0.25 and 0.175; SD, 0.07), resulting in a requirement of 16 subjects.
Considering that each subject serves as their own control and to account for potential drop-outs, a total of 50 subjects was determined as necessary for this study.
Outcome and analysis
Coprimary outcomes are (1) pupillary dilations during passive and active paradigms before and after administration of methylphenidate and apomorphine, as measured by automated pupillometry and (2) improvement in neurovascular coupling following administration of methylphenidate and apomorphine, as measured by NIRS-EEG.
Secondary outcome is an improvement of consciousness state following administration of methylphenidate and apomorphine, as measured by FOUR and SECONDs scales.
Automated pupillometry
We will assess pupillary dilations by comparing pupil sizes during passive and active paradigms to the immediate rest periods before and after administration of methylphenidate and apomorphine, as measured by automated pupillometry. Each measurement from designated segments will be compared using a Student’s t-test, with a significance threshold set at 0.05.25 50
Near-infrared spectroscopy
Our NIRS set-up features eight sources and seven detectors positioned at F1, F2, F5, F6, FC1, Fp1, Fpz, FC2, F3, F4, F7, F8, AFz, AF3 and AF4 to target the frontal areas including the prefrontal cortex, capturing neural dynamics associated with cognitive tasks and attention.51–53 To optimise our measurements, we have integrated short-distance detectors to eliminate haemodynamic extracranial interference. We will apply Monte Carlo simulations to produce a matrix detailing each channel’s spatial sensitivity to changes in cortical oxygen levels. Subsequently, we will evaluate haemodynamic baseline shifts by comparing average HbO2 levels during passive and active paradigms versus resting periods.
Electroencephalography
Using a 32-channel EEG setup, we will process and stratify the data into distinct levels of consciousness using an independent component analysis (ICA).24 Similarly, our EEG montage places emphasis on the frontal midline.53 54 In our analyses, we will particularly contrast the power of both theta (4–7 Hz) and alpha (8–12 Hz) bands between resting and task periods.
Near-infrared spectroscopy combined with electroencephalography
In our integrated neuroimaging approach, the EEG montage with high temporal sensitivity is combined with the higher spatial sensitivity of the NIRS set-up. The final step of our analysis will delve into the relationships between the neural and haemodynamic signals before and after administration of methylphenidate and apomorphine. Specifically, we will assess the neurovascular coupling by evaluating the interplay between NIRS HbO2 concentrations and EEG band-power using Wavelet cross-spectrum analysis.24
Subanalysis
Given that methylphenidate is metabolised in the liver, its plasma excretion may be affected in some ICU patients with liver failure, which can be associated with acute brain injuries or the use of drugs that alter hepatic enzyme function. To address this variable, we plan to conduct a subanalysis that adjusts for patients with liver impairments. Additionally, depending on the statistical power available, we intend to perform another subanalysis focusing on patients who receive apomorphine prior to methylphenidate. This will help rule out that the observed effects are merely carry-over effects of methylphenidate.
Data collection and management
The ICUs are screened daily for patients eligible for participating in the study trial. Patients fulfilling the criteria for inclusion are stratified into ≤UWS or ≥MCS depending on the clinical state. Once a patient is included, a stratified randomisation is conducted, and the assigned treatment order is revealed as shown in table 2.
Identification number, date and time of inclusion, clinical consciousness state, patient demographics, medical history, ongoing medication, history with occurrence of serious adverse events (SAE), cause of admission, and laboratory results are collected and entered directly into a database that complies with Danish data safety legislation. Sensitive personal data will be handled according to GDPR rules, and after the completion of the study or expiration of the permission to handle information, all data will be deleted, destructed or anonymised.
Adverse event reporting
All AEs, serious or not, occurring after the first dose of investigational product (whether attributed to investigational product), are reported immediately on the initial report form by the investigator to the sponsor. In cases of SAE, immediate reports by investigator will be made without exceeding 24 hours following knowledge of the event. Medically significant AEs considered related to the investigational product by the investigator, or the sponsor are followed until resolved or considered stable.
The relationship of an AE to investigational product will be assessed by means of an evaluation of the risks and the plausibility: ‘Is there a reasonable possibility that the event may have been caused by the investigational product?’ All study participants will be monitored closely for AE, SAE and suspected unexpected serious adverse reaction (SUSAR) until six half-lives of the active substance with longest plasma lifetime has passed. In this case, methylphenidate with 3 hours; thus 18 hours of observation before the drug can be considered ‘washed-out’ and with no relation to any subsequent AEs.
An annual safety report is submitted by the sponsor to the Danish Medicines Agency and the Ethics Committee. The report includes a listing of all suspected serious adverse reactions, which have occurred over the study period and a report of the subjects’ safety.
Ethics and dissemination
The primary objective of this study is to investigate whether apomorphine and methylphenidate can promote signatures of consciousness in unresponsive or low-responsive patients admitted to the ICU with severe brain injury. Both methylphenidate and apomorphine have undergone rigorous evaluations in clinical settings for other medical conditions, and their side effects are firmly evaluated and well documented. For instance, methylphenidate, commonly prescribed for attention deficit hyperactivity disorder (ADHD), might cause symptoms such as nausea, anxiety, tachycardia and hypertension,26–28 55 while apomorphine, used in Parkinson’s disease treatment, could lead to symptoms such as minor drowsiness, nausea, bruising from injection site or hypotension.29–32 Their adverse effects, which range from mild to moderate, are manageable, especially within the ICU setting. The likelihood of SUSAR’s occurring from the use of these medications is extremely low, as comprehensive clinical evidence has consistently shown their safety profile. It is crucial to highlight that during our study, participants will be under strict medical supervision, with comprehensive monitoring mechanisms in place to ensure their utmost safety. Therefore, the study is deemed to incur minimal risks to its participants and holds a significant potential to enhance treatment options available for patients with DoC.
The study is conducted according to The Danish law on health research in human subjects (Komitéloven) of 1 September 2020. Additional approval has been obtained from the Danish Board on Medicines and Drugs (Lægemiddelstyrelsen) according to Komitéloven, § 21.
Written informed consent is obtained by next-of-kin as well as the trial guardian according to Komitéloven, § 4, pt. 3. The decision may be withdrawn at any time without affecting the clinical treatment. However, to prevent skewed data and problems with study validity, we will retain any data that have already been sampled as well as future data related to the patient’s clinical treatment.
Positive, negative or inconclusive data will be uploaded on EudraCT, registered at clinicaltrialsregister.eu, and published in an international peer-reviewed journal according to the Declaration of Helsinki.
Discussion
The landscape of DoC is marked by complexity, uncertainty and similar profound challenges for both patients, caregivers and healthcare providers.56 57 For years, the ability to accurately detect and predict consciousness recovery in DoC patients has eluded medical science, leaving patients and their families in a state of uncertainty and despair.16 19 However, recent years have brought significant advancements in the approach to DoC treatment, specifically with the increased emphasis on pharmacological interventions.40 58 59
Despite these promising achievements, it is crucial to recognise the limitations that continue to impede progress in the search of pharmacological interventions for DoC treatment. The available evidence supporting the use of these interventions remains relatively limited, primarily based on studies involving subacute to chronic DoC patients, often characterised by small sample sizes and modest clinical effect sizes. Addressing this pressing demand requires concerted effort from researchers, healthcare professionals and policy-makers to seek innovative solutions. One solution involves the promotion of multicentre studies with cross-over designs, aimed at increasing participant numbers and evaluating multiple drugs, mitigating the challenges posed by small sample sizes and inconsistent research methods.
The present protocol outlines the first double-blinded, randomised, placebo-controlled study with a multicentre cross-over design aimed at evaluating the effects of apomorphine and methylphenidate in acute DoC patients. It also seeks to validate the accuracy and efficiency of novel technologies, such as automated pupillometry and NIRS-EEG, for detecting consciousness bedside in acute DoC patients. This allows us to investigate both clinical and proxy consciousness biomarkers. Moreover, by employing a multicentre cross-over approach, to our knowledge, we are conducting the hitherto largest clinical drug trial on apomorphine and methylphenidate in acute DoC for patients with traumatic and non-TBIs.
So far, only three pharmacological agents have been investigated in randomised clinical trials in DoC: amantadine,60–62 zolpidem63 and methylphenidate.38 Amantadine, in particular, has emerged as a recommended treatment for patients with DoC following TBI for the purpose of accelerating consciousness recovery, as per the American Academy of Neurology Guidelines for Disorders of Consciousness from 2018.64 However, in cases where patients do not respond to amantadine, alternative stimulant therapies such as methylphenidate or zolpidem are also suggested. In fact, there is a growing recognition that DoC is not a uniform condition; rather, it manifests in diverse clinical profiles and trajectories.65 This recognition has prompted a paradigm shift towards tailoring treatments based on coma endophenotypes, acknowledging that a one-size-fits-all approach may not be effective for all DoC patients. This underscores the need to explore a broader spectrum of treatment alternatives, spanning both pharmaceutical and non-pharmaceutical approaches.65
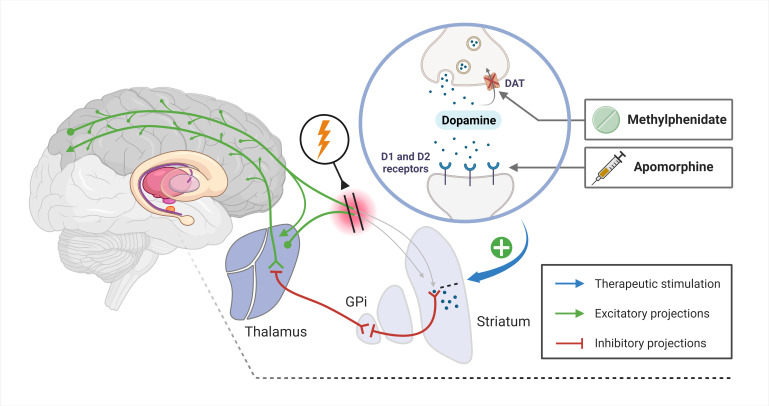
Indeed, the approach of using dopaminergic agonists to enhance arousal relies on initial findings from limited studies and case reports, but also from the mesocircuit model that offers an understanding of how dopaminergic agonists might benefit DoC patients.66 According to this model, severe brain injuries could compromise the anterior forebrain. This disruption may result in diminished dopamine levels and synaptic activity in consciousness-related areas. In particular, medium spiny neurons rely on high levels of synaptic activity and dopaminergic influences to control the internal globus pallidus. Without this control, there is a sustained inhibition of the central thalamus, leading to prolonged suppression of the anterior forebrain. Building on this theory, dopaminergic agonists such as apomorphine and methylphenidate might help restore neural connections and amplify synaptic activity, offering potential improvements for DoC patients. See figure 3 for a detailed description.
Figure 3.
Modulation of the mesocircuit model using apomorphine and methylphenidate. The mesocircuit system encompasses a loop involving the anterior forebrain, striatum, globus pallidus internus (GPi). Together with the thalamus, it plays a crucial role in regulating the cortical activity associated with consciousness. A compromised afferent drive to the striatum due to brain injury impairs the firing rates of the medium spiny neurons (MSNs). This deficit leads to an increased inhibitory output from the GPi to the thalamic nuclei, thereby weakening thalamocortical signalling.66 Apomorphine acts as a dopamine receptor agonist, directly stimulating D1 and D2 receptors,33 while methylphenidate increases dopaminergic transmission by inhibiting the reuptake of dopamine via the dopamine transporter (DAT).36 The enhancement of dopamine levels and direct receptor stimulation support MSN activity, counteracting the inhibitory imbalance within the mesocircuit and ultimately promoting the restoration of forebrain dynamics. Blue arrow denotes site of pharmacological intervention, green arrows indicate excitatory neural projections and red lines represent inhibitory neural projections within the mesocircuit. The figure is an original work created using biorender.com by the first author (MHO), who has granted permission for its reuse in this context.
Limitations
Our clinical trial aims to provide valuable insights, but it is not without limitations. Given the relatively modest effect sizes reported in current studies, truly ascertaining the impact of apomorphine and methylphenidate on clinical outcomes demands more extensive trials. Thus, we have directed our primary objective towards understanding improvements in cortical modulation of pupillary function and the neurovascular coupling of the brain following administration of apomorphine and methylphenidate. As such, our sample size determination is hinged on a detectable clinical effect size specific to these modalities. While we have made provisions for potential drop-outs, the unpredictable nature of ICU environments means our anticipated dropout rate may be conservative. As the study progresses, we might find it necessary to increase patient inclusions to account for this. Another crucial aspect to consider is the heterogeneity of our patient population. Since DoC patients can vary widely in terms of injury severity, underlying causes and individual neurophysiological responses, this could introduce significant variability in our findings. Nevertheless, while variability is a concern, the diverse population mirrors the real-world complexity of ICU settings, thereby enhancing the generalisability of our findings. Finally, patients in neurocritical care are often treated with extraventricular drains and other intracranial devices that may interfere with the placement of the NIRS-EEG cap. This could lead to a potential selection bias of enrolled patients.
In conclusion, when considering the global prevalence of DoC cases,1 expediting progress in treatment options is paramount. There persists a lack of awareness about DoC, both among the general public and within the medical community. This lack of awareness translates into insufficient funding for DoC research and limited evidence-based treatments.56 57 Through our multicentre cross-over design protocol, we intend to close the knowledge gap and pave the way for further research. Our ultimate goal is to shed light on diagnostics and treatment options for DoC, believing that such study formats can strengthen the evidence behind potential therapies.
WHO trial registration data set
Primary registry and trial identifying number
The trial has been registered at clinicaltrialsregister.eu, EudraCT Number: 2021-001453-31. Sponsor protocol number: CONMED3. The trial has been approved by the ethics committee (Journal-nr: H-21022096).
Protocol version
Protocol version 2.3. Last update was 3 October 2023.
Date of registration in primary registry
The protocol was registered at clinicaltrialsregister.eu, 3 May 2023.
Secondary identifying numbers
There are no other identifying numbers assigned to this trial.
Sources of monetary or material support
This study has received funding from Region Hovedstadens Forskningsfond, Rigshospitalets Forskningspuljer, Offerfonden (journal nr.: 19-610-00060) and Jascha Fonden in support of health care research. The funding will go to remuneration of the primary investigator’s Ph.D. programme and expenses related to medication, devices and data analysis. The contributors have no financial interest in the study. There are no conflicts of interest. The study participants receive no financial compensation.
Primary sponsor
Initiator of the study is principal investigator, DK. Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, Copenhagen, Denmark.
Secondary sponsors
There are no secondary sponsors.
Contact for public and scientific inquiries
Corresponding author and primary sponsor, DK. Please find the contact information on title page.
Recruitment status
Recruiting: participants are currently being recruited and enrolled.
Date of first enrolment
The first participant was enrolled on 12 August 2022.
Completion date
The last visit is expected to be completed before 1 June 2024.
Acknowledgments
Figures were created using biorender.com
Footnotes
Contributors: MHO is the primary investigator and has contributed to the manuscript, trial coordination, clinical trial registration and sought approvals from the Ethics Committee, Danish Medical Agency and Data Protection Authority. DK is the sponsor and principal investigator and has contributed to the theoretical framework, manuscript writing and approvals from all necessary authorities. All authors contributed to the conception and design of the study, revision of intellectual content and approval of the final version of this manuscript.
Funding: This study has received funding from Region Hovedstadens Forskningsfond, Rigshospitalets Forskningspuljer, Offerfonden and Jascha Fonden in support of health care research. The funding will go to remuneration of the primary investigator’s Ph.D. programme and expenses related to medication, devices and data analysis.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Kondziella D, Amiri M, Othman MH, et al. Incidence and prevalence of coma in the UK and the USA. Brain Commun 2022;4:fcac188. 10.1093/braincomms/fcac188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beecher HK. A definition of irreversible coma: report of the ad hoc committee of the Harvard medical school to examine the definition of brain death. JAMA 1968;205:337–40. 10.1001/jama.1968.03140320031009 [DOI] [PubMed] [Google Scholar]
- 3. MOLLARET P, GOULON M. The depassed coma (preliminary memoir). Rev Neurol (Paris) 1959;101:3–15. [PubMed] [Google Scholar]
- 4. Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58:349–53. 10.1212/wnl.58.3.349 [DOI] [PubMed] [Google Scholar]
- 5. Bruno M-A, Vanhaudenhuyse A, Thibaut A, et al. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011;258:1373–84. 10.1007/s00415-011-6114-x [DOI] [PubMed] [Google Scholar]
- 6. NHS . Disorders of consciousness, Available: https://www.nhs.uk/conditions/disorders-of-consciousness/ [Accessed 11 Sep 2023].
- 7. Bayne T, Hohwy J, Owen AM. Are there levels of consciousness. Trends Cogn Sci 2016;20:405–13. 10.1016/j.tics.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 8. Irisawa T, Vadeboncoeur TF, Karamooz M, et al. Duration of coma in out-of-hospital cardiac arrest survivors treated with targeted temperature management. Ann Emerg Med 2017;69:36–43. 10.1016/j.annemergmed.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 9. Kondziella D. Roald Dahl and the complete locked-in syndrome: ‘cold dead body, living brain. J Neurol Sci 2017;379:276–8. 10.1016/j.jns.2017.06.033 [DOI] [PubMed] [Google Scholar]
- 10. Laureys S, Pellas F, Van Eeckhout P, et al. The locked-in syndrome: what is it like to be conscious but paralyzed and voiceless Prog Brain Res 2005;150:495–511. 10.1016/S0079-6123(05)50034-7 [DOI] [PubMed] [Google Scholar]
- 11. Di Perri C, Bahri MA, Amico E, et al. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: a cross-sectional multimodal imaging study. Lancet Neurol 2016;15:830–42. 10.1016/S1474-4422(16)00111-3 [DOI] [PubMed] [Google Scholar]
- 12. Demertzi A, Jox RJ, Racine E, et al. A European survey on attitudes towards pain and end-of-life issues in locked-in syndrome. Brain Injury 2014;28:1209–15. 10.3109/02699052.2014.920526 [DOI] [PubMed] [Google Scholar]
- 13. Kondziella D. Functional neuroimaging in disorders of consciousness: raising awareness for those with decreased awareness. Neuroscience 2018;382:125–6. 10.1016/j.neuroscience.2018.03.046 [DOI] [PubMed] [Google Scholar]
- 14. Whyte J, Cifu D, Dikmen S, et al. Prediction of functional outcomes after traumatic brain injury: a comparison of 2 measures of duration of unconsciousness. Arch Phys Med Rehabil 2001;82:1355–9. 10.1053/apmr.2001.26091 [DOI] [PubMed] [Google Scholar]
- 15. Giacino JT, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome, . 1997. Available: https://philpapers.org/rec/GIATVA-2 [Accessed 11 Sep 2023].
- 16. Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009;9:35. 10.1186/1471-2377-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turgeon AF, Lauzier F, Simard J-F, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ 2011;183:1581–8. 10.1503/cmaj.101786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skibsted AP, Amiri M, Fisher PM, et al. Consciousness in neurocritical care cohort study using fMRI and EEG (CONNECT-ME): protocol for a longitudinal prospective study and a tertiary clinical care service. Front Neurol 2018;9:1012. 10.3389/fneur.2018.01012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kondziella D, Friberg CK, Frokjaer VG, et al. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016;87:485–92. 10.1136/jnnp-2015-310958 [DOI] [PubMed] [Google Scholar]
- 20. Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019;380:2497–505. 10.1056/NEJMoa1812757 [DOI] [PubMed] [Google Scholar]
- 21. Egbebike J, Shen Q, Doyle K, et al. Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: a prospective observational cohort study. Lancet Neurol 2022;21:704–13. 10.1016/S1474-4422(22)00212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Owen AM, Coleman MR, Boly M, et al. Detecting awareness in the vegetative state. Science 2006;313:1402. 10.1126/science.1130197 [DOI] [PubMed] [Google Scholar]
- 23. Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Euro J of Neurology 2020;27:741–56. 10.1111/ene.14151 Available: https://onlinelibrary.wiley.com/toc/14681331/27/5 [DOI] [PubMed] [Google Scholar]
- 24. Othman MH, Bhattacharya M, Møller K, et al. Resting-state NIRS-EEG in unresponsive patients with acute brain injury: a proof-of-concept study. Neurocrit Care 2021;34:31–44. 10.1007/s12028-020-00971-x [DOI] [PubMed] [Google Scholar]
- 25. Vassilieva A, Olsen MH, Peinkhofer C, et al. Automated pupillometry to detect command following in neurological patients: a proof-of-concept study. PeerJ 2019;7:e6929. 10.7717/peerj.6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wroblewski BA, Leary JM, Phelan AM, et al. Methylphenidate and seizure frequency in brain injured patients with seizure disorders. J Clin Psychiatry 1992;53:86–9. [PubMed] [Google Scholar]
- 27. Alban JP, Hopson MM, Ly V, et al. Effect of methylphenidate on vital signs and adverse effects in adults with traumatic brain injury. Am J Phys Med Rehabil 2004;83:131–7. 10.1097/01.phm.0000112308.68586.1d [DOI] [PubMed] [Google Scholar]
- 28. Burke DT, Glenn MB, Vesali F, et al. Effects of methylphenidate on heart rate and blood pressure among Inpatients with acquired brain injury. Am J Phys Med Rehabil 2003;82:493–7. 10.1097/01.PHM.0000073827.07072.E6 [DOI] [PubMed] [Google Scholar]
- 29. Dewey RB, Hutton JT, LeWitt PA, et al. A randomized, double-blind, placebo-controlled trial of subcutaneously injected apomorphine for parkinsonian off-state events. Arch Neurol 2001;58:1385–92. 10.1001/archneur.58.9.1385 [DOI] [PubMed] [Google Scholar]
- 30. LeWitt PA, Ondo WG, Van Lunen B, et al. Open-label study assessment of safety and adverse effects of subcutaneous apomorphine injections in treating ‘off’ episodes in advanced Parkinson disease. Clin Neuropharmacol 2009;32:89–93. 10.1097/WNF.0B013E31816D91F9 [DOI] [PubMed] [Google Scholar]
- 31. Bhidayasiri R, Garcia Ruiz PJ, Henriksen T. Practical management of adverse events related to apomorphine therapy. Parkinsonism Relat Disord 2016;33 Suppl 1:S42–8. 10.1016/j.parkreldis.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 32. Deleu D, Hanssens Y, Northway MG. Subcutaneous apomorphine: an evidence-based review of its use in Parkinson’s disease. Drugs Aging 2004;21:687–709. 10.2165/00002512-200421110-00001 [DOI] [PubMed] [Google Scholar]
- 33. Apomorphine . LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2017. [PubMed] [Google Scholar]
- 34. Fridman EA, Krimchansky BZ, Bonetto M, et al. Continuous subcutaneous apomorphine for severe disorders of consciousness after traumatic brain injury. Brain Inj 2010;24:636–41. 10.3109/02699051003610433 [DOI] [PubMed] [Google Scholar]
- 35. Fridman EA, Calvar J, Bonetto M, et al. Fast awakening from minimally conscious state with apomorphine. Brain Inj 2009;23:172–7. 10.1080/02699050802649662 [DOI] [PubMed] [Google Scholar]
- 36. Verghese C, Abdijadid S. Methylphenidate. StatPearls Publishing, 2023. [PubMed] [Google Scholar]
- 37. Reynolds JC, Rittenberger JC, Callaway CW. Methylphenidate and amantadine to stimulate reawakening in comatose patients resuscitated from cardiac arrest. Resuscitation 2013;84:818–24. 10.1016/j.resuscitation.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 38. Moein H, Khalili HA, Keramatian K. Effect of methylphenidate on ICU and hospital length of stay in patients with severe and moderate traumatic brain injury. Clin Neurol Neurosurg 2006;108:539–42. 10.1016/j.clineuro.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 39. Schnakers C, Monti MM. Disorders of consciousness after severe brain injury: therapeutic options. Curr Opin Neurol 2017;30:573–9. 10.1097/WCO.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 40. Thibaut A, Schiff N, Giacino J, et al. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol 2019;18:600–14. 10.1016/S1474-4422(19)30031-6 [DOI] [PubMed] [Google Scholar]
- 41. Edlow BL, Sanz LRD, Polizzotto L, et al. Therapies to restore consciousness in patients with severe brain injuries: a gap analysis and future directions. Neurocrit Care 2021;35(Suppl 1):68–85. 10.1007/s12028-021-01227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peinkhofer C, Martens P, Grand J, et al. Influence of strategic cortical infarctions on pupillary function. Front Neurol 2018;9:916. 10.3389/fneur.2018.00916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wijdicks EFM, Bamlet WR, Maramattom BV, et al. Validation of a new coma scale: the FOUR score. Ann Neurol 2005;58:585–93. 10.1002/ana.20611 [DOI] [PubMed] [Google Scholar]
- 44. Aubinet C, Cassol H, Bodart O, et al. Simplified evaluation of consciousness disorders (seconds) in individuals with severe brain injury: a validation study. Ann Phys Rehabil Med 2021;64:101432. 10.1016/j.rehab.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 45. Platt RW, Schisterman EF, Cole SR. Time-modified confounding. Am J Epidemiol 2009;170:687–94. 10.1093/aje/kwp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Y-L, Shi L, Agbo F, et al. LC-MS/MS simultaneous quantification of apomorphine and its major metabolites in human plasma: application to clinical comparative bioavailability evaluation for the apomorphine sublingual film and a subcutaneous product. J Pharm Biomed Anal 2020;190:113493. 10.1016/j.jpba.2020.113493 [DOI] [PubMed] [Google Scholar]
- 47. Ang ZY, Boddy M, Liu Y, et al. Stability of apomorphine in solutions containing selected antioxidant agents. Drug Des Devel Ther 2016;10:3253–65. 10.2147/DDDT.S116848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 49. Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and BIOMEDICAL sciences. Behav Res Methods 2007;39:175–91. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- 50. Othman MH, Olsen MH, Møller K, et al. Automated Pupillometry to Uncover Signs of Consciousness in Acute Brain Injury: Statistical Analysis Plan. 1 August 2022. 10.5281/ZENODO.6627565 [DOI] [Google Scholar]
- 51. Yeung MK, Lee TL, Han YMY, et al. Prefrontal activation and pupil dilation during N-back task performance: a combined fNIRS and pupillometry study. Neuropsychologia 2021;159. 10.1016/j.neuropsychologia.2021.107954 [DOI] [PubMed] [Google Scholar]
- 52. Pfurtscheller G, Bauernfeind G, Wriessnegger SC, et al. Focal frontal (De)Oxyhemoglobin responses during simple arithmetic. Int J Psychophysiol 2010;76:186–92. 10.1016/j.ijpsycho.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 53. Shin J, Müller KR, Hwang HJ. Eyes-closed hybrid brain-computer interface employing frontal brain activation. PLoS One 2018;13:e0196359. 10.1371/journal.pone.0196359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gärtner M, Grimm S, Bajbouj M. Frontal midline theta oscillations during mental arithmetic: effects of stress. Front Behav Neurosci 2015;9:96. 10.3389/fnbeh.2015.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Volkow ND, Wang GJ, Gatley SJ, et al. Temporal relationships between the pharmacokinetics of methylphenidate in the human brain and its behavioral and cardiovascular effects. Psychopharmacology (Berl) 1996;123:26–33. 10.1007/BF02246277 [DOI] [PubMed] [Google Scholar]
- 56. Claassen J, Akbari Y, Alexander S, et al. Proceedings of the first Curing Coma Campaign NIH symposium: challenging the future of research for coma and disorders of consciousness. Neurocrit Care 2021;35:4–23. 10.1007/s12028-021-01260-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Provencio JJ, Hemphill JC, Claassen J, et al. The curing coma campaign: framing initial scientific challenges-proceedings of the first curing coma campaign scientific advisory council meeting. Neurocrit Care 2020;33:1–12. 10.1007/s12028-020-01028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kondziella D, Amiri M, Othman MH, et al. Understanding, detecting, and stimulating consciousness recovery in the ICU. Acta Neurochir (Wien) 2023;165:809–28. 10.1007/s00701-022-05378-5 [DOI] [PubMed] [Google Scholar]
- 59. Edlow BL, Sanz LRD, Polizzotto L, et al. Therapies to restore consciousness in patients with severe brain injuries: A gap analysis and future directions. Neurocrit Care 2021;35:68–85. 10.1007/s12028-021-01227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giacino JT, Whyte J, Bagiella E, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med 2012;366:819–26. 10.1056/NEJMoa1102609 [DOI] [PubMed] [Google Scholar]
- 61. Hintze TD, Small CE, Montgomery J, et al. Comparison of amantadine, modafinil, and standard of care in the acute treatment of disorders of consciousness after severe traumatic brain injury. Clin Neuropharmacol 2022;45:1–6. 10.1097/WNF.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 62. Shafiee S, Ehteshami S, Moosazadeh M, et al. Placebo-controlled trial of oral amantadine and zolpidem efficacy on the outcome of patients with acute severe traumatic brain injury and diffuse axonal injury. Caspian J Intern Med 2022;13:113–21. 10.22088/cjim.13.1.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Whyte J, Rajan R, Rosenbaum A, et al. Zolpidem and restoration of consciousness. Am J Phys Med Rehabil 2014;93:101–13. 10.1097/PHM.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 64. Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology; the American Congress of rehabilitation medicine. Arch Phys Med Rehabil 2018;99:1699–709. 10.1016/j.apmr.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 65. Kondziella D, Menon DK, Helbok R, et al. A precision medicine framework for classifying patients with disorders of consciousness: advanced classification of consciousness endotypes (ACCESS). Neurocrit Care 2021;35(Suppl 1):27–36. 10.1007/s12028-021-01246-9 [DOI] [PubMed] [Google Scholar]
- 66. Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci 2010;33:1–9. 10.1016/j.tins.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sergi PG, Bilotta F. Plum and Posner’s diagnosis and treatment of stupor and coma. Anesth Analg 2020;131:e15–6. 10.1213/ANE.0000000000004832 [DOI] [Google Scholar]
- 68. Kondziella D, Frontera JA. Pearls & Oy-Sters: eyes-open coma. Neurology 2021;96:864–7. 10.1212/WNL.0000000000011715 [DOI] [PubMed] [Google Scholar]
- 69. Laureys S, Celesia GG, Cohadon F, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 2010;8:1–4. 10.1186/1741-7015-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nakase-Richardson R, Yablon SA, Sherer M, et al. Serial yes/no reliability after traumatic brain injury: implications regarding the operational criteria for emergence from the minimally conscious state. J Neurol Neurosurg Psychiatry 2008;79:216–8. 10.1136/jnnp.2007.127795 [DOI] [PubMed] [Google Scholar]