Abstract
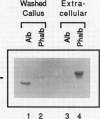
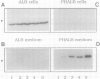
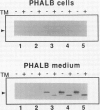
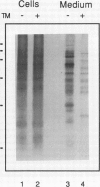
A cytosolic pea (Pisum sativum) seed albumin (ALB) and a chimeric protein (PHALB) consisting of the signal peptide and first three amino acids of phytohemagglutinin (PHA) and the amino acid sequence of ALB were expressed in parallel suspension cultures of tobacco (Nicotiana tabacum) cells and their intracellular fates examined. PHALB was efficiently secreted by the cells whereas ALB remained intracellular. These experiments show that the information contained in the signal peptide of a vacuolar protein is both necessary and sufficient for efficient secretion, and define secretion as a default or bulk-flow pathway. Entry into the secretory pathway was accompanied by glycosylation and the efficient conversion of the high mannose glycans into complex glycans indicating that transported glycoproteins do not need specific recognition domains for the modifying enzymes in the Golgi. Tunicamycin depressed the accumulation of the unglycosylated polypeptide in the culture medium much less than the accumulation of other glycoproteins. We interpret this as evidence that glycans on proteins that are not normally glycosylated do not have the same function of stabilizing and protecting the polypeptide as on natural glycoproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiol. 1985 Oct;79(2):568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranski T. J., Faust P. L., Kornfeld S. Generation of a lysosomal enzyme targeting signal in the secretory protein pepsinogen. Cell. 1990 Oct 19;63(2):281–291. doi: 10.1016/0092-8674(90)90161-7. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Denecke J., Botterman J., Deblaere R. Protein secretion in plant cells can occur via a default pathway. Plant Cell. 1990 Jan;2(1):51–59. doi: 10.1105/tpc.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel C., Voelker T. A., Herman E. M., Chrispeels M. J. Transport of proteins to the plant vacuole is not by bulk flow through the secretory system, and requires positive sorting information. J Cell Biol. 1989 Feb;108(2):327–337. doi: 10.1083/jcb.108.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Apparent Inhibition of beta-Fructosidase Secretion by Tunicamycin May Be Explained by Breakdown of the Unglycosylated Protein during Secretion. Plant Physiol. 1989 Mar;89(3):845–851. doi: 10.1104/pp.89.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye L., Sturm A., Bollini R., Vitale A., Chrispeels M. J. The position of the oligosaccharide side-chains of phytohemagglutinin and their accessibility to glycosidases determines their subsequent processing in the Golgi. Eur J Biochem. 1986 Aug 1;158(3):655–661. doi: 10.1111/j.1432-1033.1986.tb09803.x. [DOI] [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Tunicamycin inhibits protein glycosylation in suspension cultured soybean cells. Plant Physiol. 1981 May;67(5):882–886. doi: 10.1104/pp.67.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- Kaushal G. P., Elbein A. D. Glycoprotein processing enzymes of plants. Methods Enzymol. 1989;179:452–475. doi: 10.1016/0076-6879(89)79146-1. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lund P., Lee R. Y., Dunsmuir P. Bacterial chitinase is modified and secreted in transgenic tobacco. Plant Physiol. 1989 Sep;91(1):130–135. doi: 10.1104/pp.91.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesch A., Hartmann E., Rohde K., Rubartelli A., Sitia R., Rapoport T. A. A novel pathway for secretory proteins? Trends Biochem Sci. 1990 Mar;15(3):86–88. doi: 10.1016/0968-0004(90)90186-f. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Sijmons P. C., Dekker B. M., Schrammeijer B., Verwoerd T. C., van den Elzen P. J., Hoekema A. Production of correctly processed human serum albumin in transgenic plants. Biotechnology (N Y) 1990 Mar;8(3):217–221. doi: 10.1038/nbt0390-217. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Williamson M. S., Forde J., Buxton B., Kreis M. Nucleotide sequence of barley chymotrypsin inhibitor-2 (CI-2) and its expression in normal and high-lysine barley. Eur J Biochem. 1987 May 15;165(1):99–106. doi: 10.1111/j.1432-1033.1987.tb11199.x. [DOI] [PubMed] [Google Scholar]