Abstract
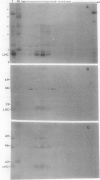
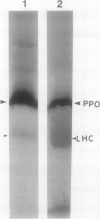
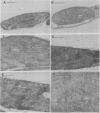
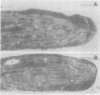
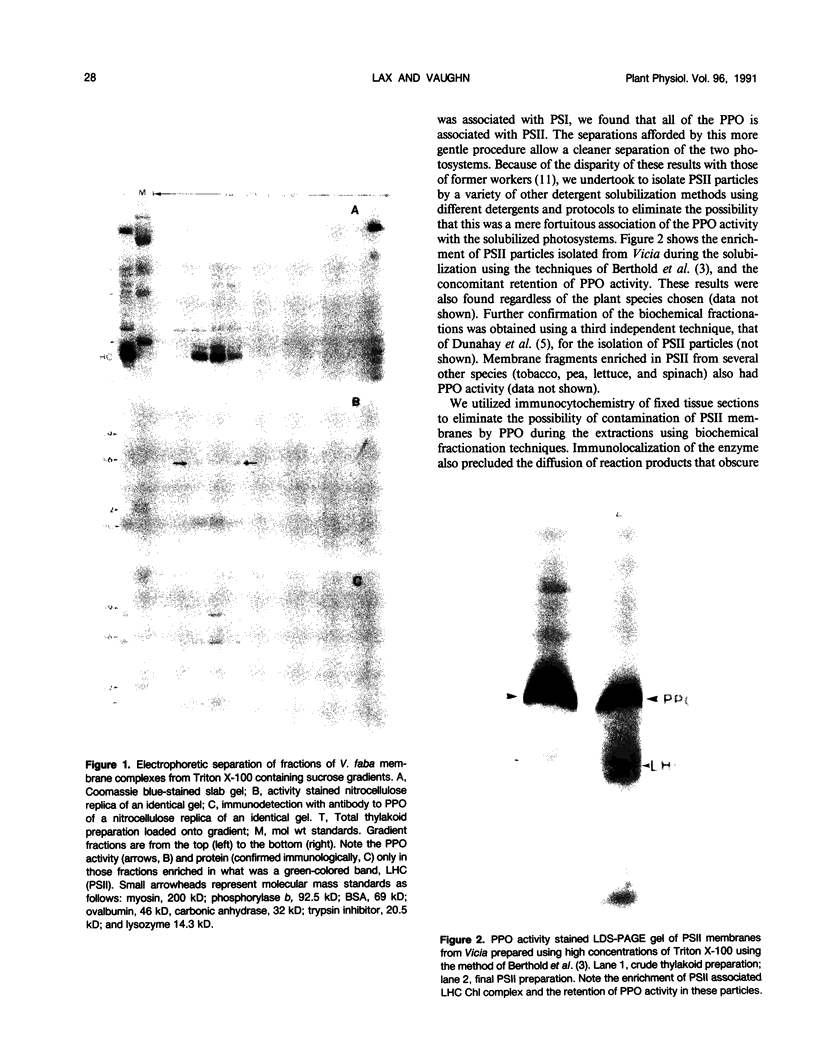
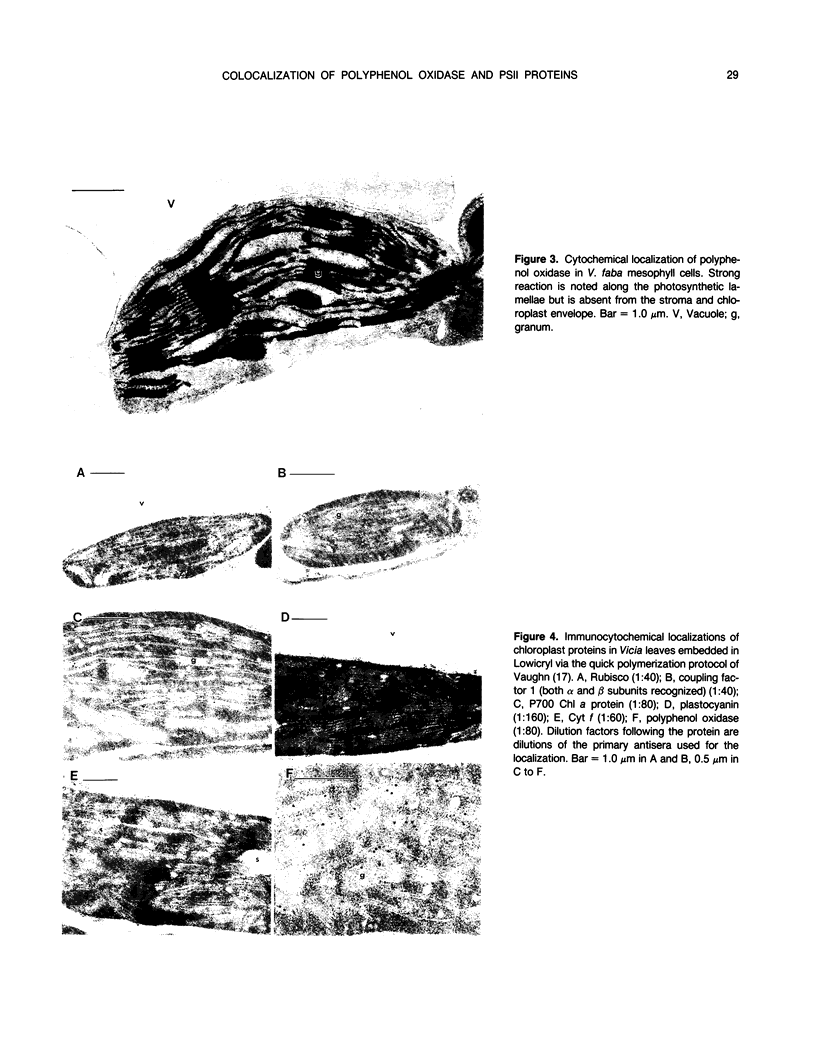
Polyphenol oxidase (PPO) appears to be ubiquitous in higher plants but, as yet, no function has been ascribed to it. Herein, we report on the localization of PPO based upon biochemical fractionation of chloroplast membranes in Vicia faba (broad bean) into various complexes and immunocytochemical electron microscopic investigations. Sucrose density gradient fractionations of thylakoid membranes after detergent solubilization reveals that PPO protein (by reactivity with anti-PPO antibody) and activity (based upon ability to oxidize di-dihydroxyphenylalanine) are found only in fractions enriched in photosystem II (PSII). Furthermore, of the PSII particles isolated using three different protocols utilizing several plant species, all had PPO. Immunogold localization of PPO on thin sections reveals exclusive thylakoid labeling with a distribution pattern consistent with other PSII proteins (80% grana, 20% stroma). These data strongly indicate that PPO is at least peripherally associated with the PSII complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. R., Staehelin L. A. Lateral Distribution of the Cytochrome b(6)/f and Coupling Factor ATP Synthetase Complexes of Chloroplast Thylakoid Membranes. Plant Physiol. 1985 May;78(1):199–202. doi: 10.1104/pp.78.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey W. H. Polypeptide composition and amino-terminal sequence of broad bean polyphenoloxidase. Plant Physiol. 1989 Oct;91(2):481–483. doi: 10.1104/pp.91.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gol'dfel'd M. G., Khalilov R. I. Lokalizatsiia medi v fotosinteticheskom apparate khloroplasta. Biofizika. 1979 Jul-Aug;24(4):762–764. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Staehelin L. A. Analysis of the thylakoid outer surface. Coupling factor is limited to unstacked membrane regions. J Cell Biol. 1976 Jan;68(1):30–47. doi: 10.1083/jcb.68.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Activation of polyphenol oxidase of chloroplasts. Plant Physiol. 1973 Feb;51(2):234–244. doi: 10.1104/pp.51.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]