Abstract
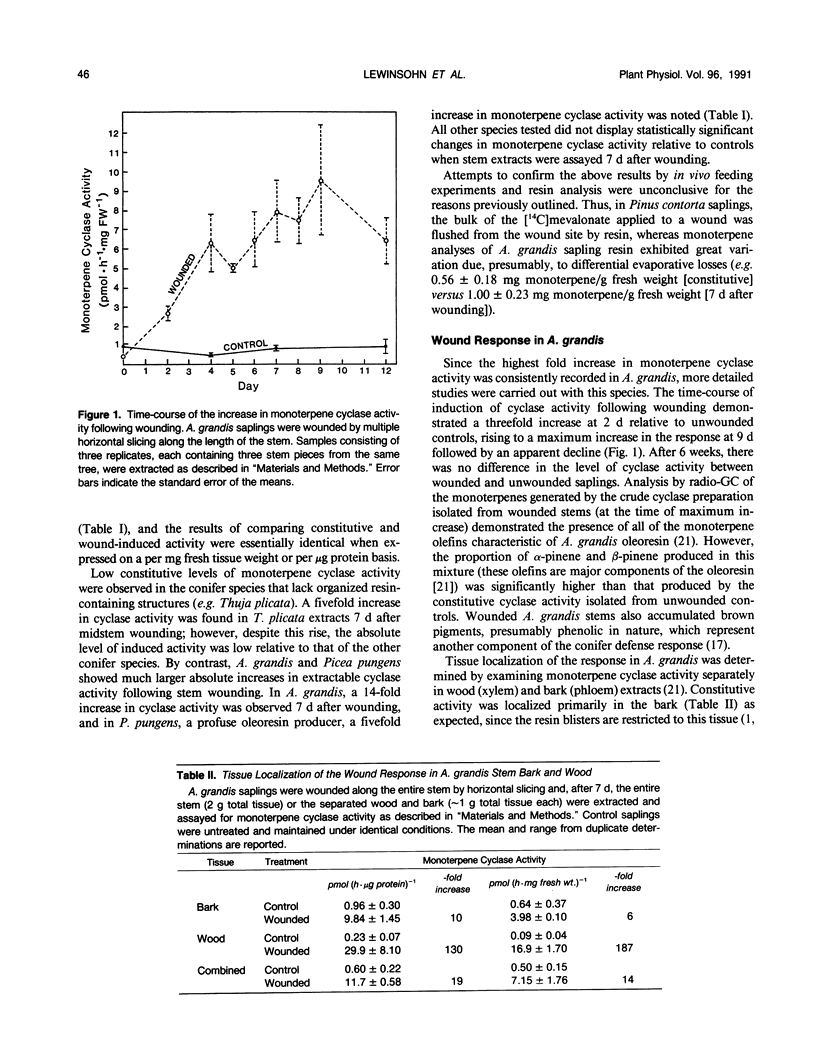
Levels of monoterpene cyclase activity were determined in extracts from wounded and unwounded saplings of 10 conifer species to assess whether oleoresin biosynthesis is induced by stem wounding. Species of Abies and Picea, with low to moderate levels of constitutive monoterpene cyclase activity, exhibited a five- to 15-fold increase in cyclase activity 7 days after wounding relative to unwounded controls. In contrast, species of genera such as Pinus, with high levels of constitutive cyclase activity, did not significantly respond to wounding by alteration in the level of cyclase activity. The highest fold increase in monoterpene cyclase activity was consistently observed in Abies grandis, and the time-course of induction of activity following stem wounding in this species demonstrated a threefold increase at 2 days relative to unwounded controls, rising to a maximum increase in the response at 9 days (greater than 10-fold) followed by an apparent decline. The wound response was localized, and both bark (phloem) and wood (xylem) tissues displayed increased cyclase activity at the wound site. The magnitude of the increase in cyclase activity was dependent on the severity of the wound.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dueber M. T., Adolf W., West C. A. Biosynthesis of the Diterpene Phytoalexin Casbene: Partial Purification and Characterization of Casbene Synthetase from Ricinis communis. Plant Physiol. 1978 Oct;62(4):598–603. doi: 10.1104/pp.62.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E., Gijzen M., Savage T. J., Croteau R. Defense mechanisms of conifers : relationship of monoterpene cyclase activity to anatomical specialization and oleoresin monoterpene content. Plant Physiol. 1991 May;96(1):38–43. doi: 10.1104/pp.96.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 1988 Dec;88(4):1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]


