Abstract
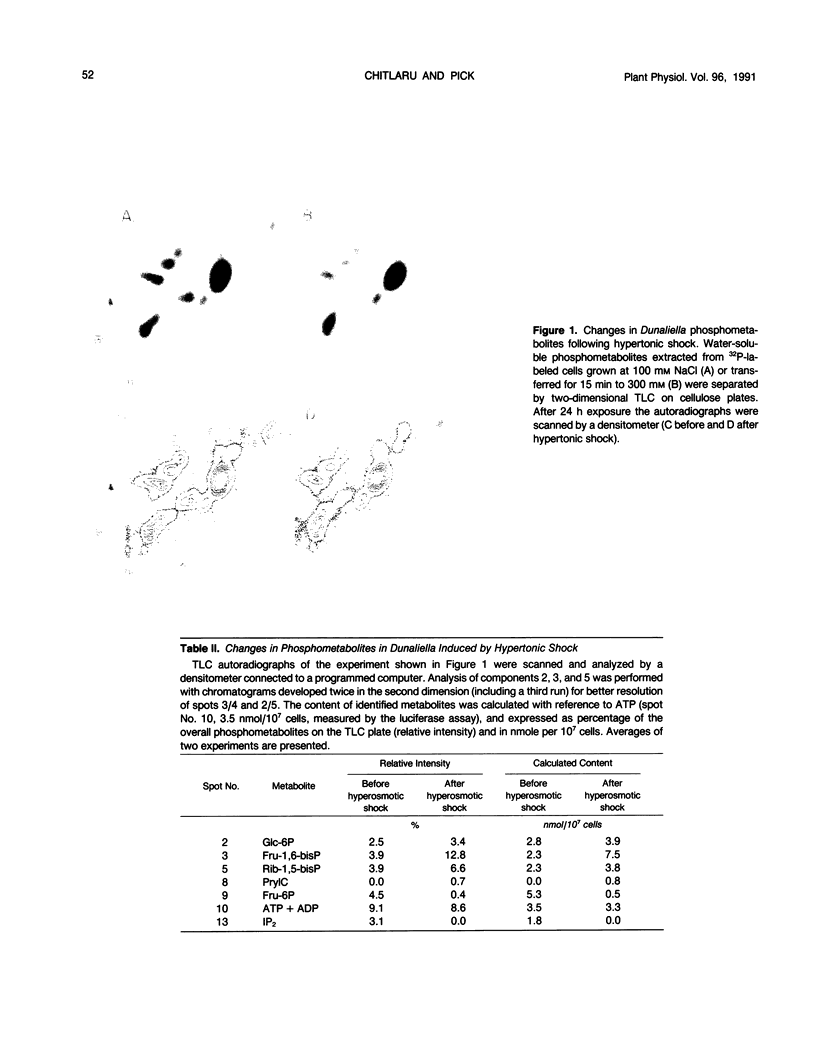
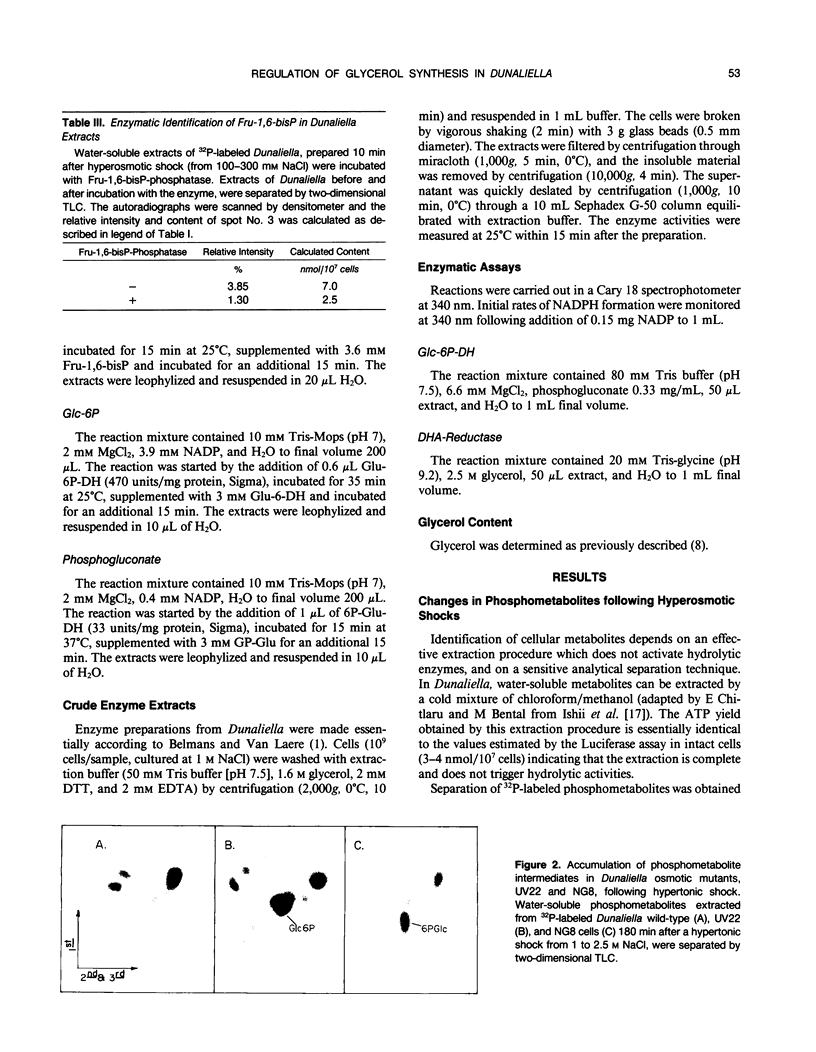
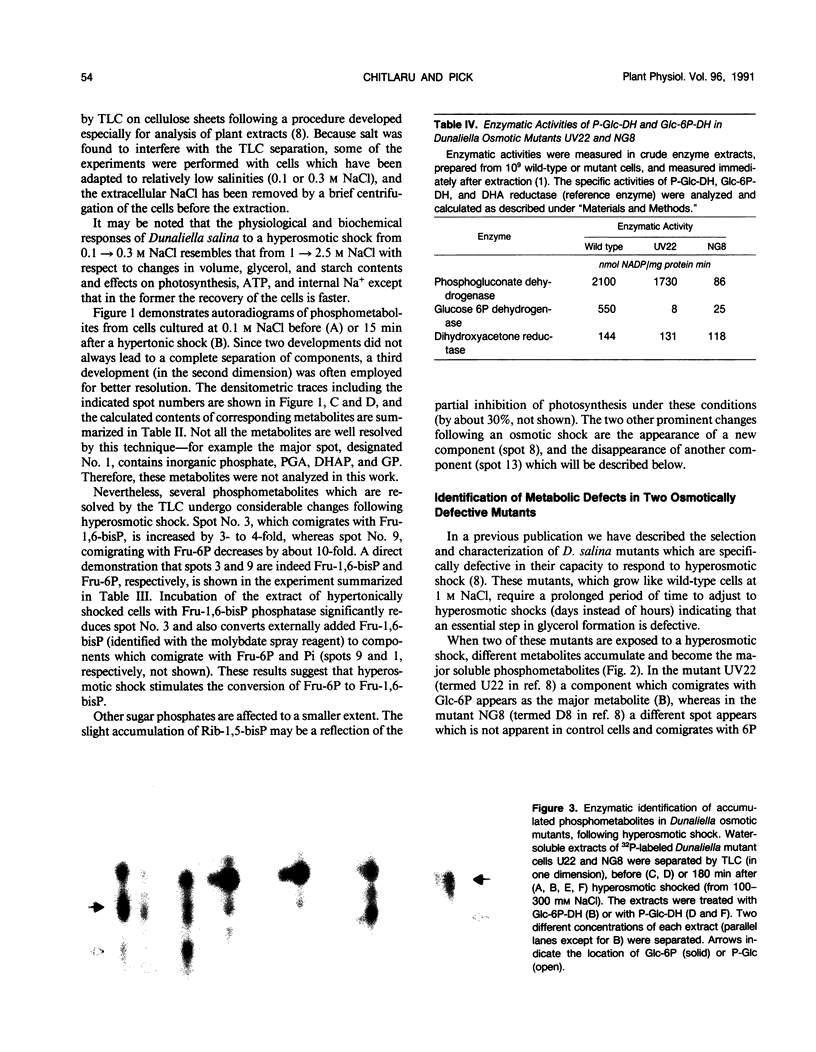
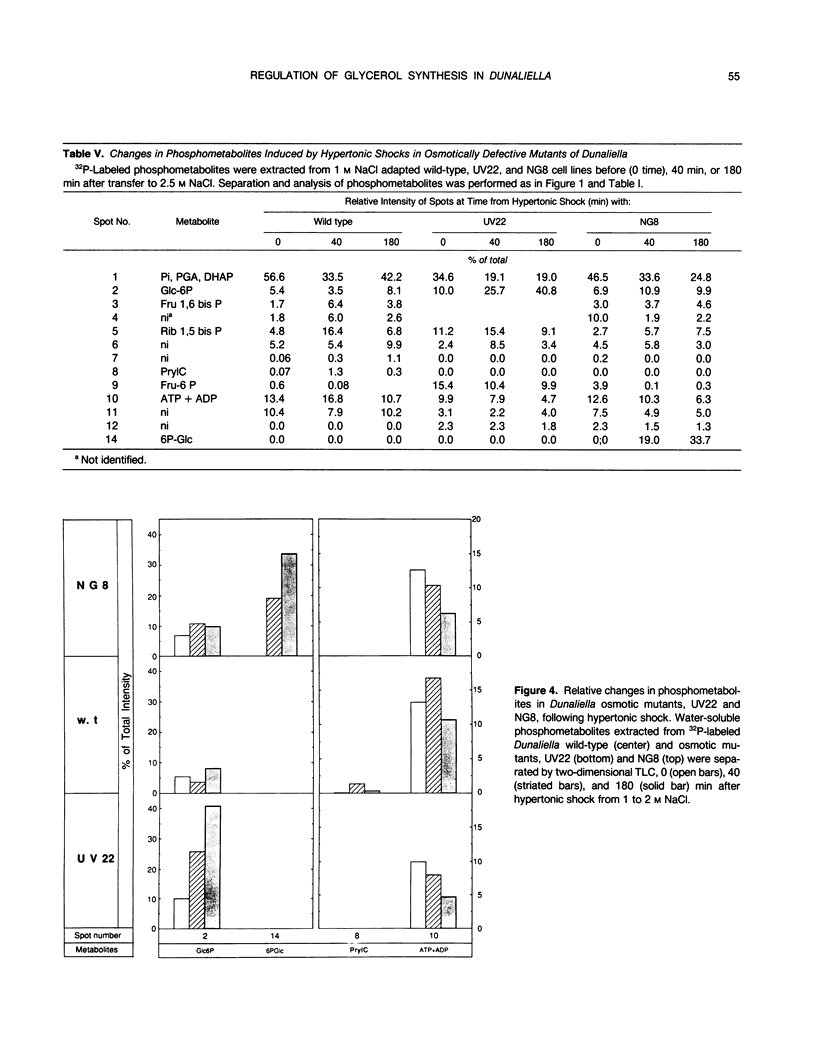
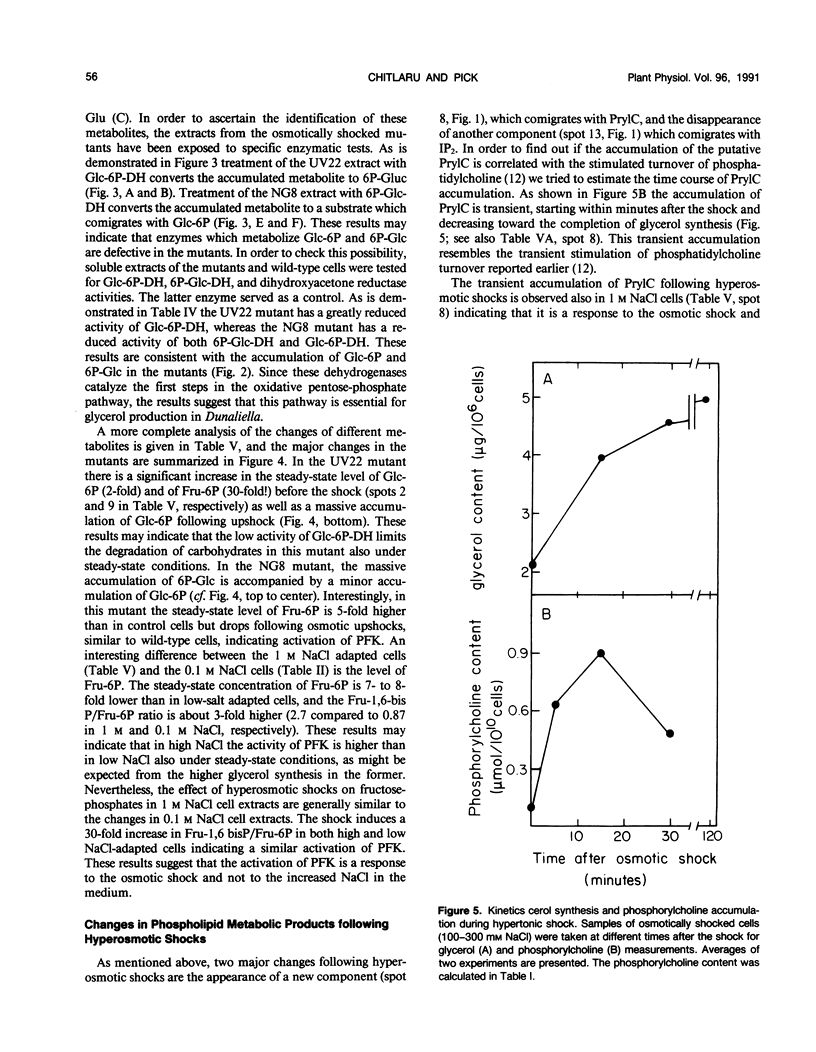
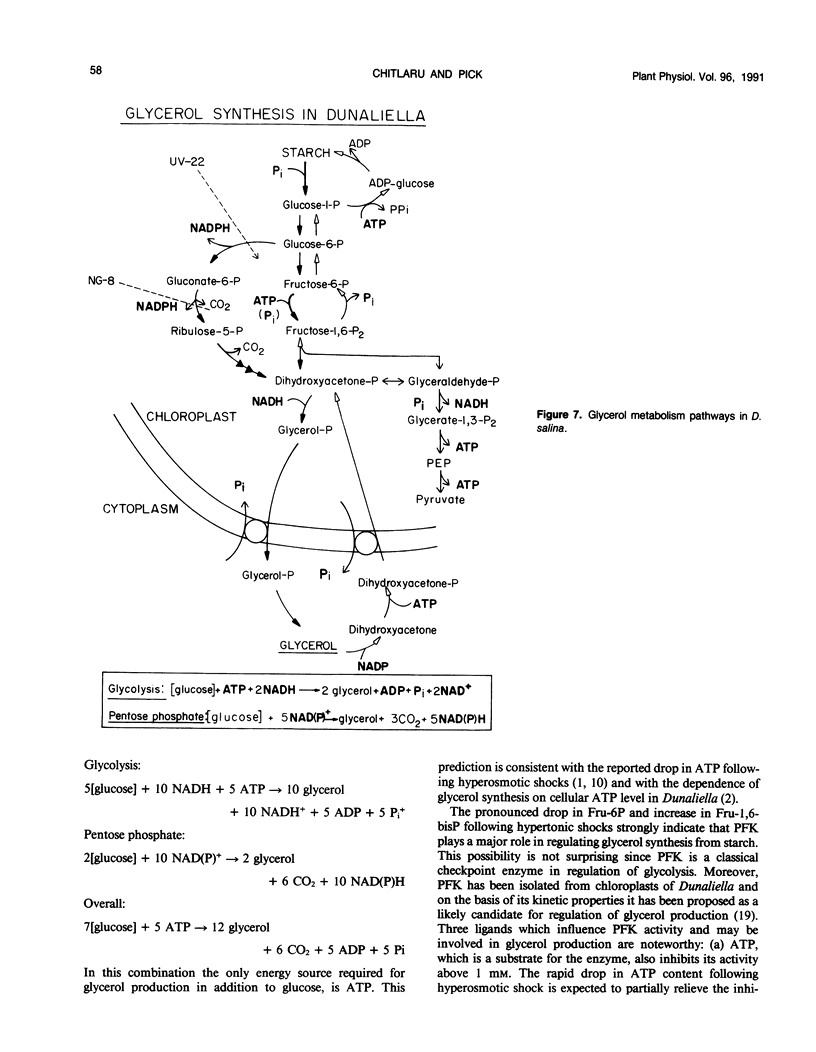
Changes in phosphometabolites, following osmotic shock, were analyzed by two-dimensional thin layer chromatography, in extracts of the halotolerant alga Dunaliella salina in order to clarify the regulation of glycerol synthesis from starch. The experiments were carried out in wild-type and in osmotically defective mutant cells. It is demonstrated that hyperosmotic shock induces a decrease in fructose 6-phosphate and an increase in fructose-1,6-bisphosphate indicating the activation of phosphofructokinase. Two mutants, which are specifically defective in their response to hyperosmotic shock, accumulate glucose 6-phosphate or phosphogluconate following shock, and have remarkably reduced activities of glucose-6-phosphate dehydrogenase and of phosphogluconate dehydrogenase, respectively. These results indicate that the pentose-phosphate oxidative pathway has a major role in glycerol synthesis. Hyperosmotic shock leads to a transient accumulation of phosphorylcholine and to a decrease of inositolbisphosphate in D. salina extracts. Accumulation of phosphorylcholine is not detected in osmotically defective mutants. Hypoosmotic shock induces an increase in inositolbisphosphate but not in phosphorylcholine. These results are consistent with previous indications for differential activations of phospholipases by hyper or hypoosmotic shock in Dunaliella. Based on these results we suggest that (a) phosphofructokinase is an important checkpoint enzyme in the regulation of glycerol production, and (b) that the pentose-phosphate pathway has a major role in keeping oxidation-reduction balance during glycerol synthesis. The possible role of lipid breakdown products as second messengers in regulating glycerol production in Dunaliella is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Amotz A., Avron M. The Role of Glycerol in the Osmotic Regulation of the Halophilic Alga Dunaliella parva. Plant Physiol. 1973 May;51(5):875–878. doi: 10.1104/pp.51.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bental M., Pick U., Avron M., Degani H. The role of intracellular orthophosphate in triggering osmoregulation in the alga Dunaliella salina. Eur J Biochem. 1990 Feb 22;188(1):117–122. doi: 10.1111/j.1432-1033.1990.tb15378.x. [DOI] [PubMed] [Google Scholar]
- Borowitzka L. J., Brown A. D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch Mikrobiol. 1974 Mar 1;96(1):37–52. doi: 10.1007/BF00590161. [DOI] [PubMed] [Google Scholar]
- Chitlaru E., Pick U. Selection and Characterization of Dunaliella salina Mutants Defective in Haloadaptation. Plant Physiol. 1989 Oct;91(2):788–794. doi: 10.1104/pp.91.2.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Maeda M., Thompson G. A., Jr Concurrent changes in Dunaliella salina ultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol. 1988 Aug;107(2):529–538. doi: 10.1083/jcb.107.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Peeler T. C., Thompson G. A., Jr Rapid changes in polyphosphoinositide metabolism associated with the response of Dunaliella salina to hypoosmotic shock. J Biol Chem. 1988 Apr 25;263(12):5775–5779. [PubMed] [Google Scholar]
- Feige B., Gimmler H., Jeschke W. D., Simonis W. Eine Methode zur dünnschichtchromatographischen Auftrennung von 14C- und 32P-markierten Stoffwechselprodukten. J Chromatogr. 1969 Apr 22;41(1):80–90. doi: 10.1016/0021-9673(64)80099-6. [DOI] [PubMed] [Google Scholar]
- Ishii H., Connolly T. M., Bross T. E., Majerus P. W. Inositol cyclic triphosphate [inositol 1,2-(cyclic)-4,5-triphosphate] is formed upon thrombin stimulation of human platelets. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6397–6401. doi: 10.1073/pnas.83.17.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Wöber G. Chloroplast phosphofructokinase in the green alga, Dunaliella marina: partial purification and kinetic and regulatory properties. Arch Biochem Biophys. 1982 Feb;213(2):602–619. doi: 10.1016/0003-9861(82)90590-2. [DOI] [PubMed] [Google Scholar]
- Oren-Shamir M., Pick U., Avron M. Plasma membrane potential of the alga dunaliella, and its relation to osmoregulation. Plant Physiol. 1990 Jun;93(2):403–408. doi: 10.1104/pp.93.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]