Abstract
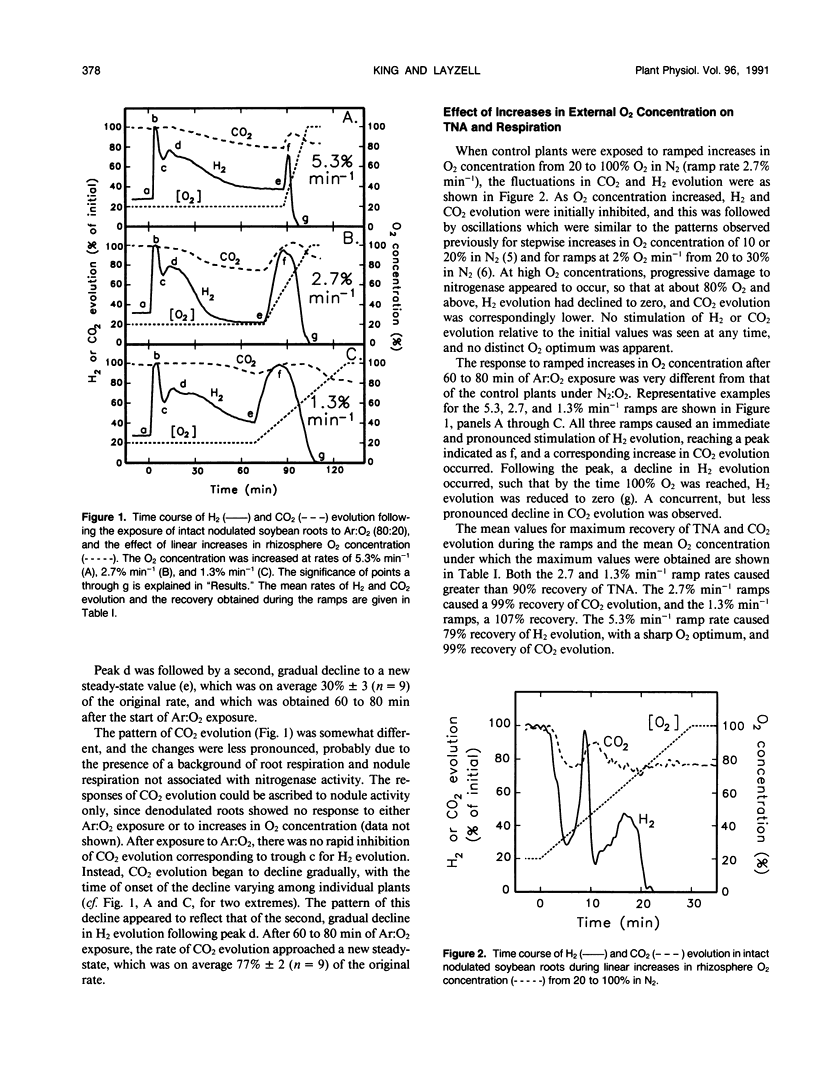
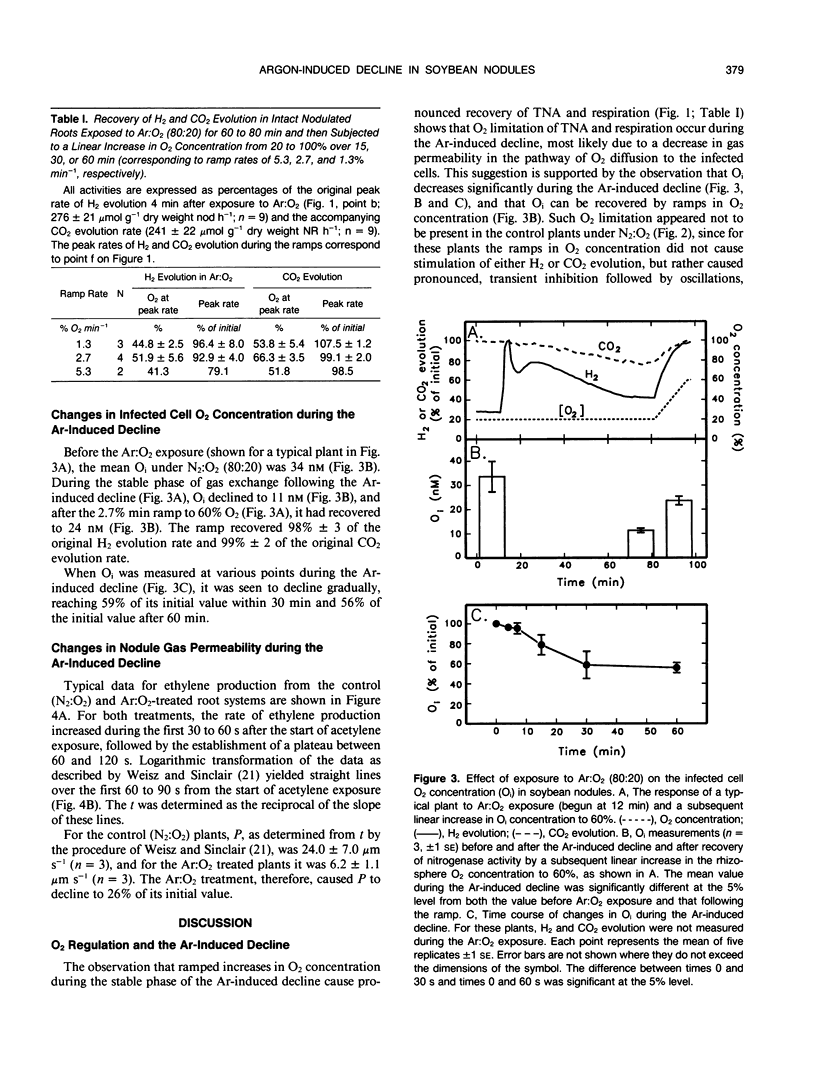
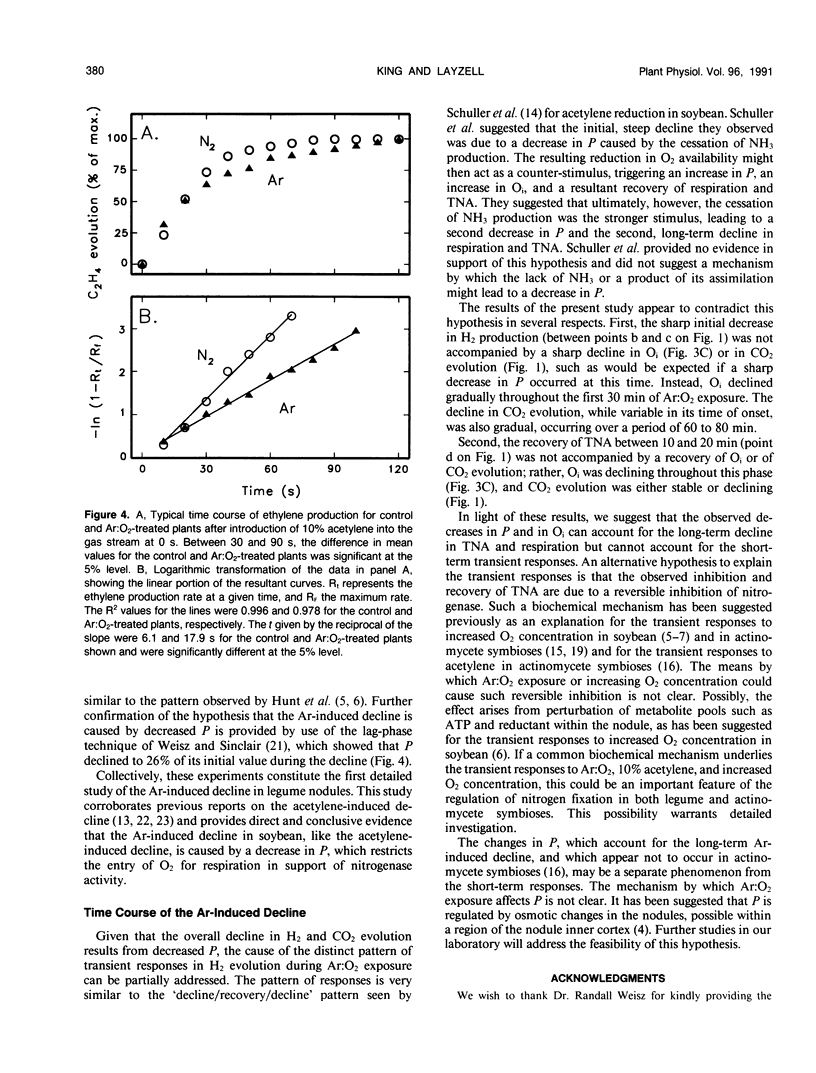
When intact nodulated roots of soybean (Glycine max L. Merr. nodulated with Bradyrhizobium japonicum strain USDA 16) were exposed to an atmosphere lacking N2 gas (Ar:O2 80:20), total nitrogenase activity (measured as H2 evolution) and respiration (CO2 evolution) declined with time of exposure. In Ar-inhibited nodules, when the O2 concentration in the rhizosphere was increased in a linear `ramp' of 2.7% per minute, 93% of the original H2 evolution and 99% of the CO2 evolution could be recovered. The internal nodule O2 concentration (estimated from leghemoglobin oxygenation) declined to 56% of its initial value after 60 minutes of Ar:O2 exposure and could be partially recovered by the linear increases in O2 concentration. Nodule gas permeability, as estimated from the lag in ethylene production following exposure of nodules to acetylene, decreased to 26% of its initial value during the Ar-induced decline. Collectively, the results provide direct evidence that the Ar-induced decline results from decreased nodule gas permeability and indicate that the decline in permeability, rather than being immediate, occurs gradually over the period of Ar:O2 exposure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hunt S., King B. J., Canvin D. T., Layzell D. B. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987 May;84(1):164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S., King B. J., Layzell D. B. Effects of gradual increases in o(2) concentration on nodule activity in soybean. Plant Physiol. 1989 Sep;91(1):315–321. doi: 10.1104/pp.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Hunt S., Weagle G. E., Walsh K. B., Pottier R. H., Canvin D. T., Layzell D. B. Regulation of o(2) concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988 Jun;87(2):296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Hunt S., Palmer G. R. Mechanism of Nitrogenase Inhibition in Soybean Nodules : Pulse-Modulated Spectroscopy Indicates that Nitrogenase Activity Is Limited by O(2). Plant Physiol. 1990 Apr;92(4):1101–1107. doi: 10.1104/pp.92.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Weagle G. E., Canvin D. T. A highly sensitive, flow through h(2) gas analyzer for use in nitrogen fixation studies. Plant Physiol. 1984 Jul;75(3):582–585. doi: 10.1104/pp.75.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvester W. B., Winship L. J. Transient responses of nitrogenase to acetylene and oxygen in actinorhizal nodules and cultured frankia. Plant Physiol. 1990 Feb;92(2):480–486. doi: 10.1104/pp.92.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkema J. D., Schwintzer C. R., Monz C. A. Time course of acetylene reduction in nodules of five actinorhizal genera. Plant Physiol. 1988 Feb;86(2):581–583. doi: 10.1104/pp.86.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


