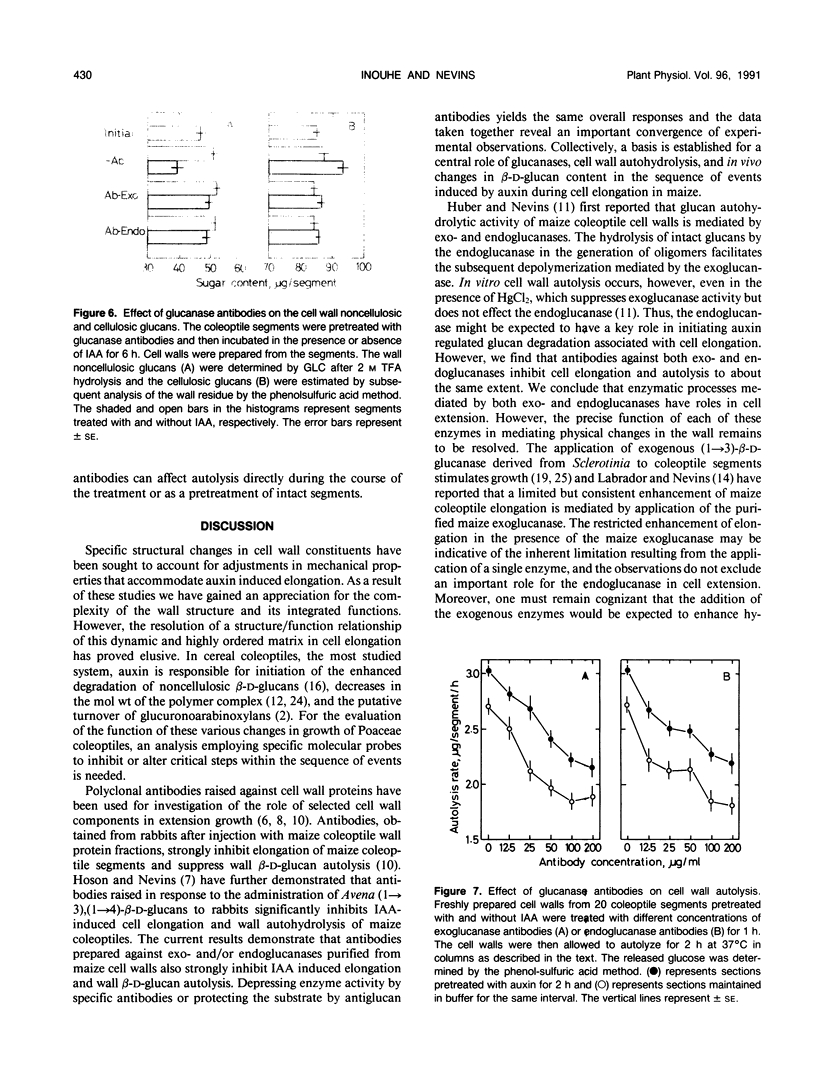
Abstract
Polyclonal antibodies were raised in rabbits in response to the administration of purified exo- and endoglucanases extracted from cell walls of maize (Zea mays L. B37 × Mo17) coleoptiles. Since the antibodies formed specific conjugates when challenged with the glucanase antigens in immunoblot assays they were employed to evaluate the participation of glucanases in tissue growth. Indole-3-acetic acid induced cell elongation of abraded coleoptile segments was inhibited when the antibodies were supplied as a short term pretreatment (25-200 microgram/milliliter of serum protein). The extent of inhibition of IAA induced cell elongation was additive when endo- and exoglucanase antibodies were applied together. The results suggest that both enzymes have a role in mediating IAA-induced cell elongation. Pretreatment with exo- and endoglucanases antibodies also inhibited IAA induced degradation of noncellulosic β-d-glucans and the increased level of cellulosic polymers in maize coleoptiles. Antibodies also inhibited the expression of the autohydrolytic degradation of glucans in isolated cell walls. The extent of inhibition was dependent on the antibody concentration applied. The results support the contention that enzymatic processes mediated by exo- and endoglucanases are responsible for cell wall autolytic reactions and that these reactions are linked to the mechanism for expressing auxin induced cell elongation in maize coleoptiles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Della-Penna D., Christoffersen R. E., Bennett A. B. Biotinylated proteins as molecular weight standards on Western blots. Anal Biochem. 1986 Feb 1;152(2):329–332. doi: 10.1016/0003-2697(86)90417-3. [DOI] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. beta-d-Glucan Hydrolase Activity in Zea Coleoptile Cell Walls. Plant Physiol. 1980 May;65(5):768–773. doi: 10.1104/pp.65.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouhe M., Nevins D. J. Auxin-enhanced glucan autohydrolysis in maize coleoptile cell walls. Plant Physiol. 1991 May;96(1):285–290. doi: 10.1104/pp.96.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttenegger D. G., Nevins D. J. Transient Nature of a (1 --> 3), (1 --> 4)-beta-d-Glucan in Zea mays Coleoptile Cell Walls. Plant Physiol. 1985 Jan;77(1):175–178. doi: 10.1104/pp.77.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y., Yamamoto R. Effect of auxin on beta-1, 3-glucanase activity in Avena coleoptile. Dev Growth Differ. 1970 Mar;11(4):287–296. doi: 10.1111/j.1440-169x.1970.00287.x. [DOI] [PubMed] [Google Scholar]