Abstract
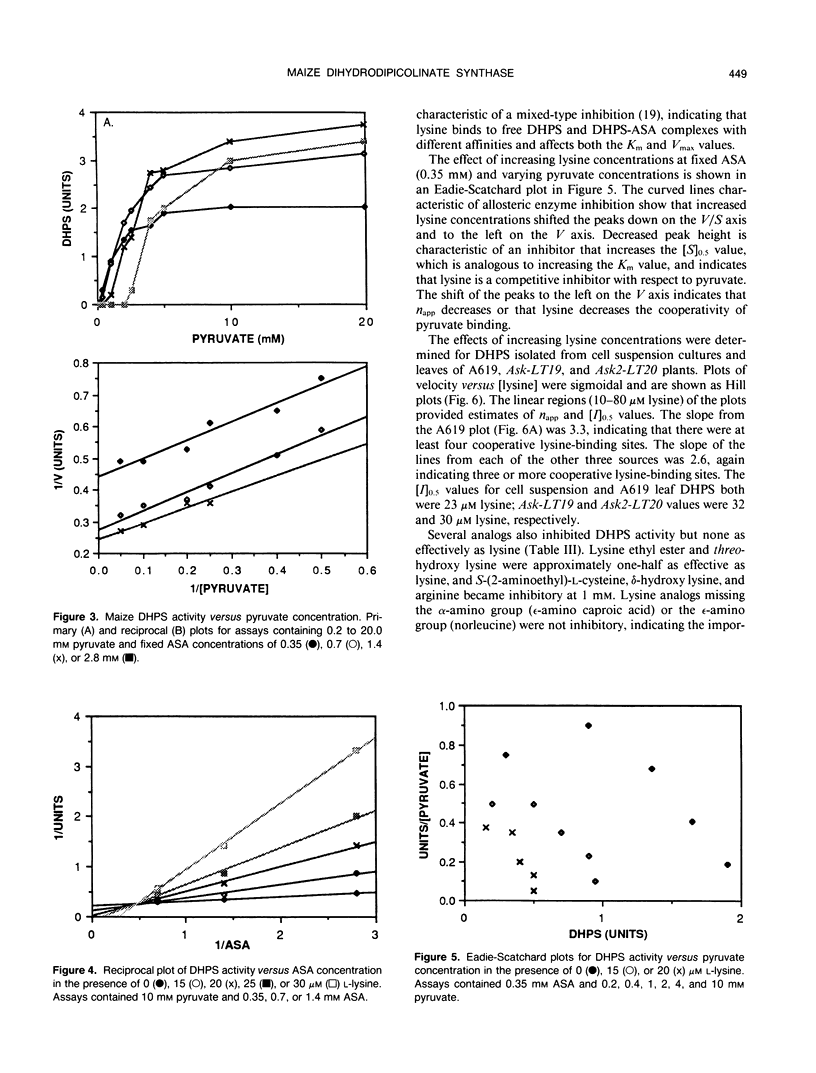
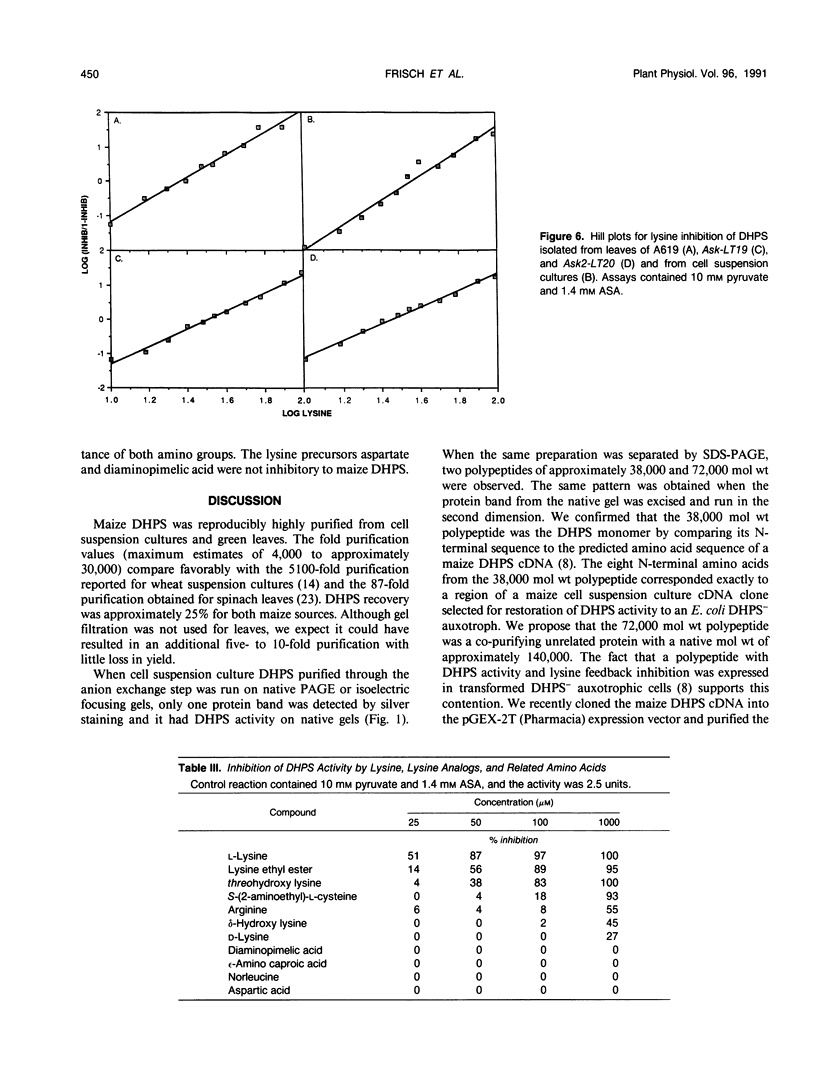
Dihydrodipicolinate synthase (EC 4.2.1.52), the first enzyme specific to lysine biosynthesis in plants, was purified from maize (Zea mays L.) cell suspension cultures and leaves. The subunit molecular weight of maize dihydrodipicolinate synthase was estimated to be 38,000 based on SDS-PAGE. The condensation of l-aspartate semialdehyde and pyruvate by highly purified dihydrodipicolinate synthase exhibited kinetics characteristic of a Ping Pong Bi Bi ordered reaction in which pyruvate binds first to the enzyme. Substrate inhibition evident at higher concentrations of l-aspartate semialdehyde was partially alleviated by increasing concentrations of pyruvate. Pyruvate binding exhibited cooperativity with an apparent number of 2 and 1.86 millimolar concentration required for 50% of maximal activity. The Km for aspartate semialdehyde was estimated to be 0.6 millimolar concentration. Lysine was an allosteric cooperative inhibitor of dihydrodipicolinate synthase with an estimated Hill number of 4 and 23 micromolar concentration required for 50% inhibition. The physical and kinetic data are consistent with a homotetramer model for the native enzyme.
Full text
PDF

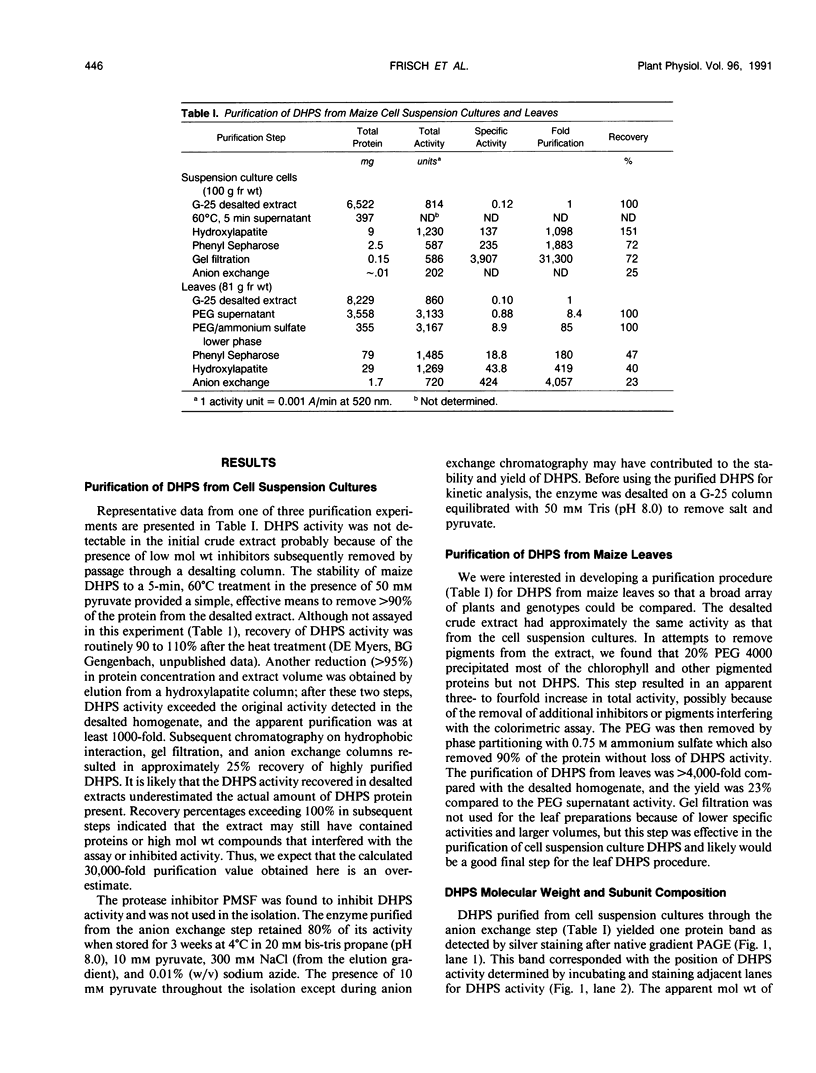
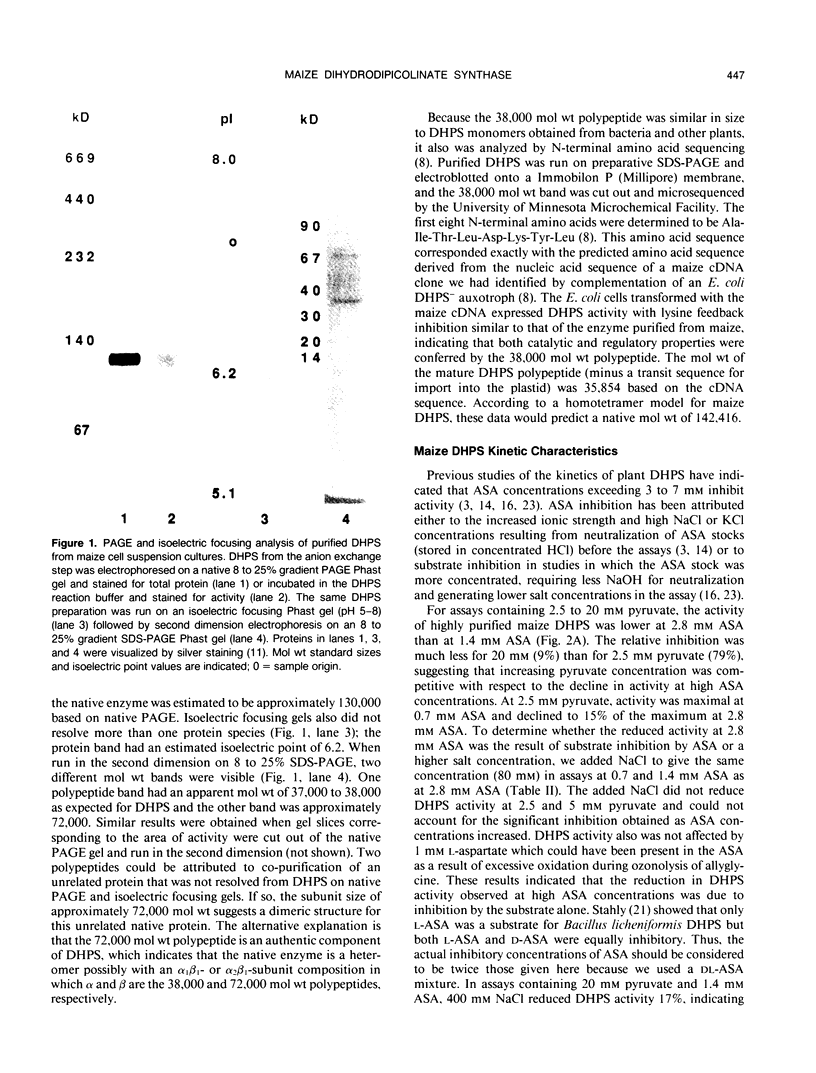
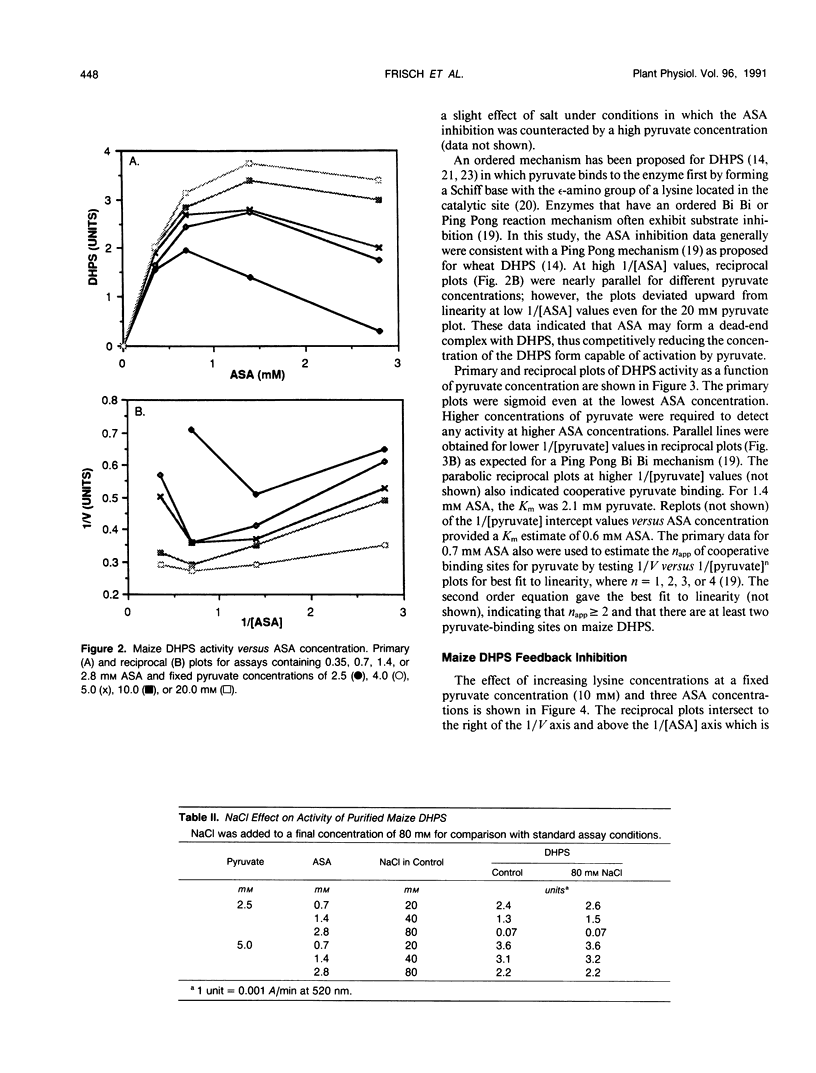


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., WRIGHT N. G. Aspartic beta-semialdehyde dehydrogenase and aspartic beta-semialdehyde. J Biol Chem. 1955 Mar;213(1):39–50. [PubMed] [Google Scholar]
- Dotson S. B., Somers D. A., Gengenbach B. G. Kinetic studies of lysine-sensitive aspartate kinase purified from maize suspension cultures. Plant Physiol. 1990 May;93(1):98–104. doi: 10.1104/pp.93.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson S. B., Somers D. A., Gengenbach B. G. Purification and characterization of lysine-sensitive aspartate kinase from maize cell cultures. Plant Physiol. 1989 Dec;91(4):1602–1608. doi: 10.1104/pp.91.4.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Stahly D. P. Dihydrodipicolinic acid synthase of Bacillus licheniformis. Quaternary structure, kinetics, and stability in the presence of sodium chloride and substrates. Biochim Biophys Acta. 1976 Dec 8;452(2):580–596. doi: 10.1016/0005-2744(76)90209-6. [DOI] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988 Jan;9(1):28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- Hibberd K. A., Green C. E. Inheritance and expression of lysine plus threonine resistance selected in maize tissue culture. Proc Natl Acad Sci U S A. 1982 Jan;79(2):559–563. doi: 10.1073/pnas.79.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Hashimoto T., Kumpaisal R., Yamada Y. Molecular cloning of wheat dihydrodipicolinate synthase. J Biol Chem. 1990 Oct 15;265(29):17451–17455. [PubMed] [Google Scholar]
- Kumpaisal R., Hashimoto T., Yamada Y. Purification and characterization of dihydrodipicolinate synthase from wheat suspension cultures. Plant Physiol. 1987 Sep;85(1):145–151. doi: 10.1104/pp.85.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelis M., Whatley F. R., Whatley J. The enzymology of lysine biosynthesis in higher plants. The occurrence, characterization and some regulatory properties of dihydrodipicolinate synthase. FEBS Lett. 1977 Dec 15;84(2):236–240. doi: 10.1016/0014-5793(77)80696-0. [DOI] [PubMed] [Google Scholar]
- Richaud F., Richaud C., Ratet P., Patte J. C. Chromosomal location and nucleotide sequence of the Escherichia coli dapA gene. J Bacteriol. 1986 Apr;166(1):297–300. doi: 10.1128/jb.166.1.297-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlarski J. G., Gilvarg C. The pyruvate-aspartic semialdehyde condensing enzyme of Escherichia coli. J Biol Chem. 1970 Mar 25;245(6):1362–1373. [PubMed] [Google Scholar]
- Stahly D. P. Dihydrodipicolinic acid synthase of Bacillus licheniformis. Biochim Biophys Acta. 1969 Nov 4;191(2):439–451. doi: 10.1016/0005-2744(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Mazelis M. The enzymology of lysine biosynthesis in higher plants: complete localization of the regulatory enzyme dihydrodipicolinate synthase in the chloroplasts of spinach leaves. FEBS Lett. 1980 Jul 28;116(2):189–192. doi: 10.1016/0014-5793(80)80640-5. [DOI] [PubMed] [Google Scholar]
- Walter T. J., Connelly J. A., Gengenbach B. G., Wold F. Isolation and characterization of two homoserine dehydrogenases from maize suspension cultures. J Biol Chem. 1979 Feb 25;254(4):1349–1355. [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Yamakura F., Ikeda Y., Kimura K., Sasakawa T. Partial purification and some properties of pyruvate-aspartic semialdehyde condensing enzyme from sporulating Bacillus subtilis. J Biochem. 1974 Sep;76(3):611–621. doi: 10.1093/oxfordjournals.jbchem.a130605. [DOI] [PubMed] [Google Scholar]