Abstract
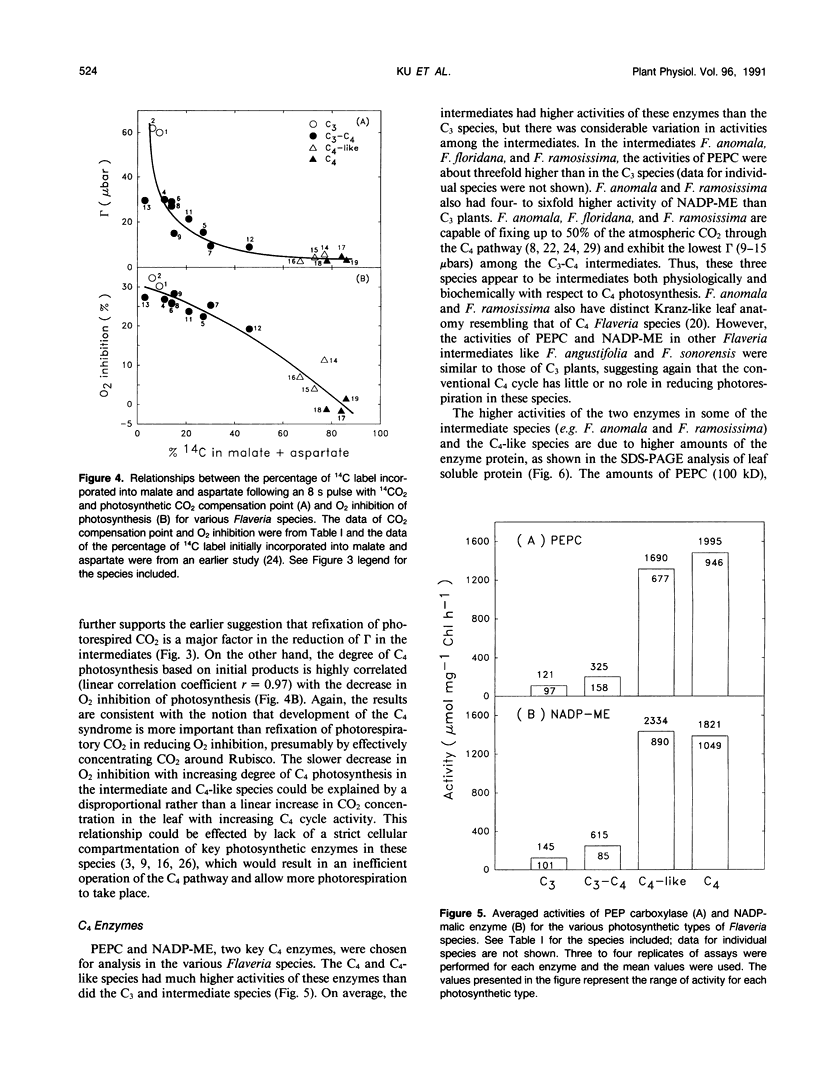
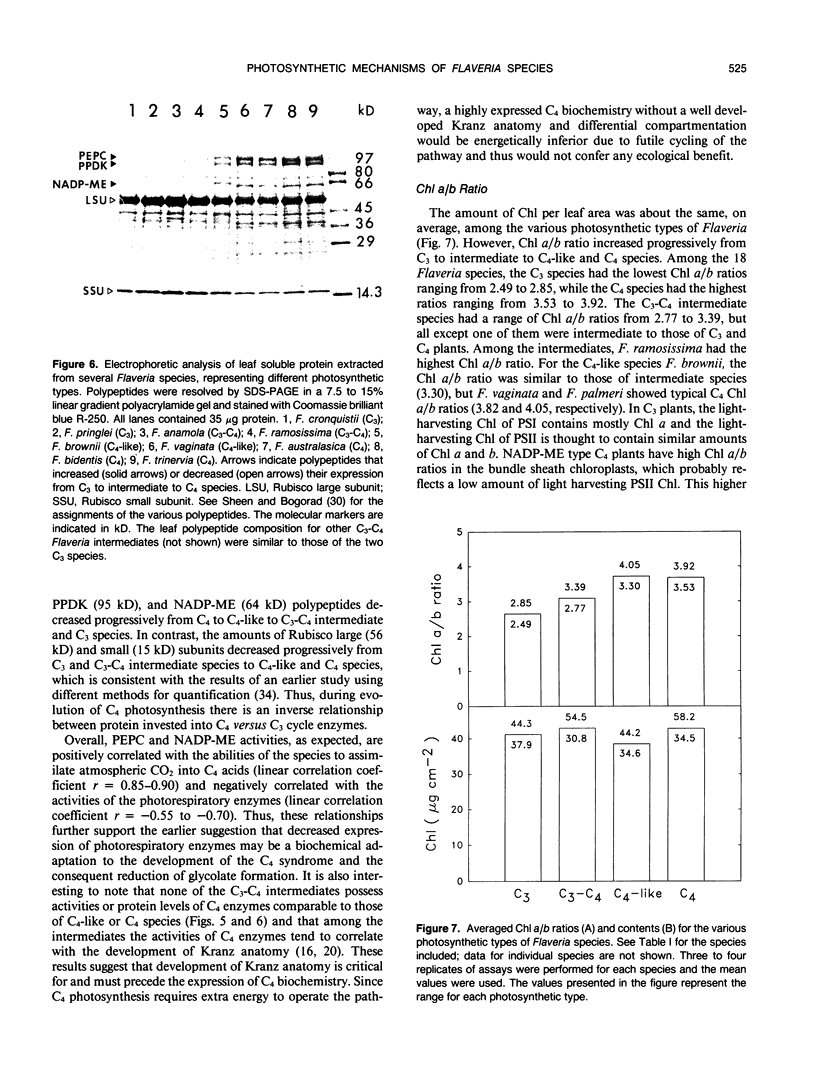
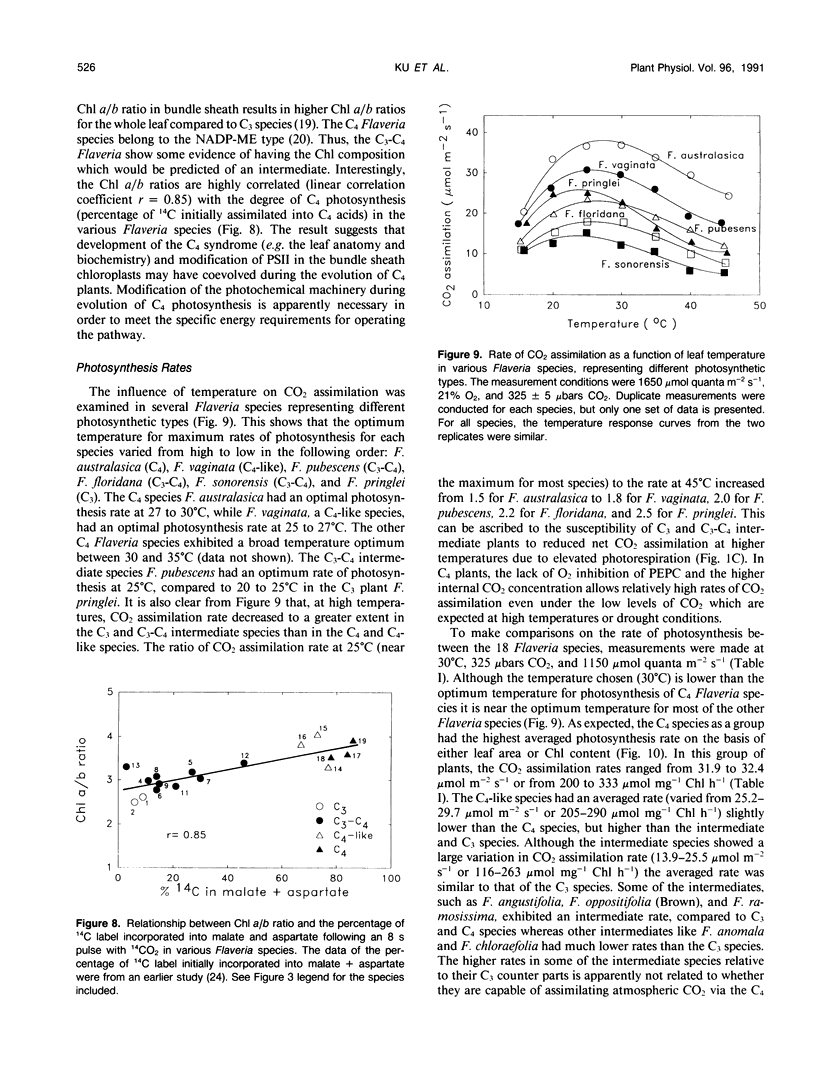
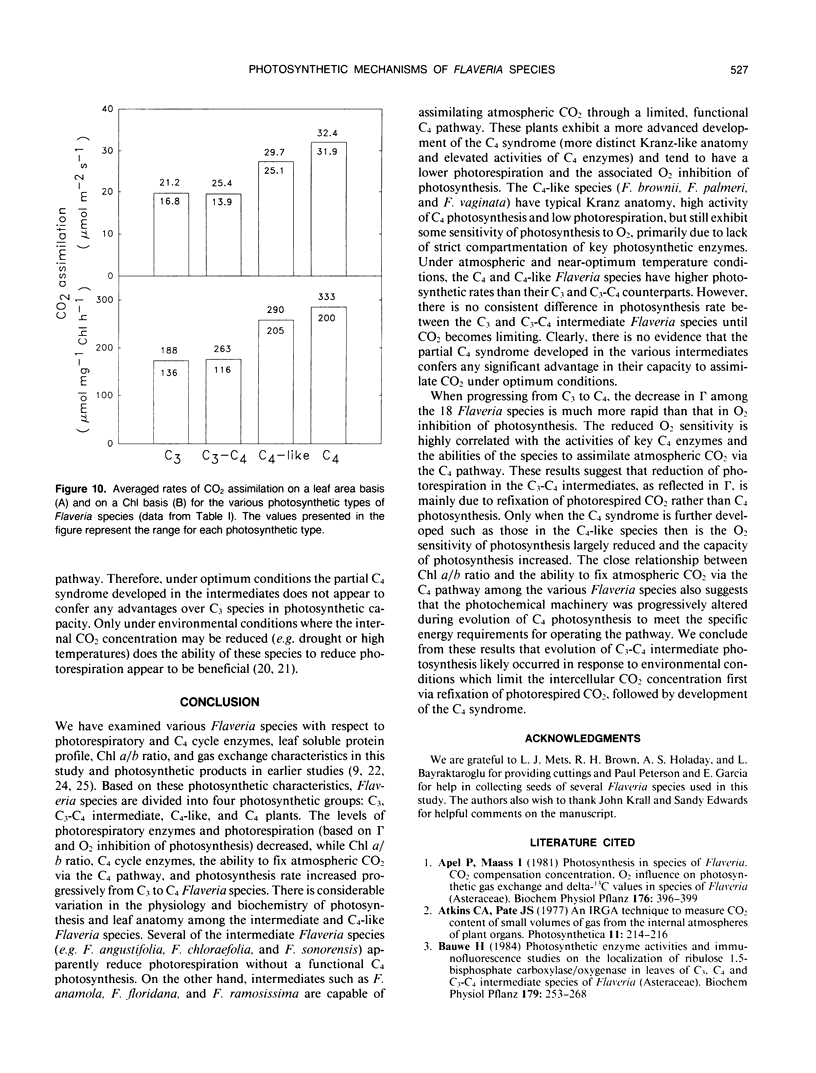
The genus Flaveria shows evidence of evolution in the mechanism of photosynthesis as its 21 species include C3, C3-C4, C4-like, and C4 plants. In this study, several physiological and biochemical parameters of photosynthesis and photorespiration were measured in 18 Flaveria species representing all the photosynthetic types. The 10 species classified as C3-C4 intermediates showed an inverse continuum in level of photorespiration and development of the C4 syndrome. This ranges from F. sonorensis with relatively high apparent photorespiration and lacking C4 photosynthesis to F. Among the intermediates, the photosynthetic CO2 compensation points at 30°C and 1150 micromoles quanta per square meter per second varied from 9 to 29 microbars. The values for the three C4-like species varied from 3 to 6 microbars, similar to those measured for the C4 species. The activities of the photorespiratory enzymes glycolate oxidase, hydroxypyruvate reductase, and serine hydroxymethyltransferase decreased progressively from C3 to C3-C4 to C4-like and C4 species. On the other hand, most intermediates had higher levels of phosphenolpyruvate carboxylase and NADP-malic enzyme than C3 species, but generally lower activities compared to C4-like and C4 species. The levels of these C4 enzymes are correlated with the degree of C4 photosynthesis, based on the initial products of photosynthesis. Another indication of development of the C4 syndrome in C3-C4 Flaveria species was their intermediate chlorophyll a/b ratios. The chlorophyll a/b ratios of the various Flaveria species are highly correlated with the degree of C4 photosynthesis suggesting that the photochemical machinery is progressively altered during evolution in order to meet the specific energy requirements for operating the C4 pathway. In the progression from C3 to C4 species in Flaveria, the CO2 compensation point decreased more rapidly than did the decrease in O2 inhibition of photosynthesis or the increase in the degree of C4 photosynthesis. These results suggest that the reduction in photorespiration during evolution occurred initially by refixation of photorespired CO2 and prior to substantive reduction in O2 inhibition and development of the C4 syndrome. However, further reduction in O2 inhibition in some intermediates and C4-like species is considered primarily due to the development of the C4 syndrome. Thus, the evolution of C3-C4 intermediate photosynthesis likely occurred in response to environmental conditions which limit the intercellular CO2 concentration first via refixation of photorespired CO2, followed by development of the C4 syndrome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauwe H., Chollet R. Kinetic properties of phosphoenolpyruvate carboxylase from c(3), c(4), and c(3)-c(4) intermediate species of flaveria (asteraceae). Plant Physiol. 1986 Nov;82(3):695–699. doi: 10.1104/pp.82.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Hattersley P. W. Leaf anatomy of c(3)-c(4) species as related to evolution of c(4) photosynthesis. Plant Physiol. 1989 Dec;91(4):1543–1550. doi: 10.1104/pp.91.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H., Morgan J. A. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways : VI. DIFFERENTIAL EFFECTS OF TEMPERATURE AND LIGHT INTENSITY ON PHOTORESPIRATION IN C(3), C(4), AND INTERMEDIATE SPECIES. Plant Physiol. 1980 Oct;66(4):541–544. doi: 10.1104/pp.66.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Moore B. D., Edwards G. E., Ku M. S. Photosynthesis in Flaveria brownii, a C(4)-Like Species: Leaf Anatomy, Characteristics of CO(2) Exchange, Compartmentation of Photosynthetic Enzymes, and Metabolism of CO(2). Plant Physiol. 1988 Aug;87(4):867–873. doi: 10.1104/pp.87.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Moore B. D., Wu J., Edwards G. E., Ku M. S. Photosynthetic Plasticity in Flaveria brownii: Growth Irradiance and the Expression of C(4) Photosynthesis. Plant Physiol. 1989 Apr;89(4):1129–1135. doi: 10.1104/pp.89.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Dai Z., Ku M. S., Edwards G. E. Induction of Crassulacean Acid Metabolism in the Facultative Halophyte Mesembryanthemum crystallinum by Abscisic Acid. Plant Physiol. 1990 Jul;93(3):1253–1260. doi: 10.1104/pp.93.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Holaday A. S., Brown R. H., Bartlett J. M., Sandlin E. A., Jackson R. C. Enzymic and Photosynthetic Characteristics of Reciprocal F(1) Hybrids of Flaveria pringlei (C(3)) and Flaveria brownii (C(4)-Like Species). Plant Physiol. 1988 Jun;87(2):484–490. doi: 10.1104/pp.87.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M. S., Monson R. K., Littlejohn R. O., Nakamoto H., Fisher D. B., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : I. Leaf Anatomy, Photosynthetic Responses to O(2) and CO(2), and Activities of Key Enzymes in the C(3) and C(4) Pathways. Plant Physiol. 1983 Apr;71(4):944–948. doi: 10.1104/pp.71.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson R. K., Schuster W. S., Ku M. S. Photosynthesis in Flaveria brownii A.M. Powell : A C(4)-Like C(3)-C(4) Intermediate. Plant Physiol. 1987 Dec;85(4):1063–1067. doi: 10.1104/pp.85.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C. B., Harris B. Photorespiration during C 4 photosynthesis. Biochim Biophys Acta. 1971 May 11;234(2):270–282. doi: 10.1016/0005-2728(71)90082-x. [DOI] [PubMed] [Google Scholar]
- Rumpho M. E., Ku M. S., Cheng S. H., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : III. Reduction of Photorespiration by a Limited C(4) Pathway of Photosynthesis in Flaveria ramosissima. Plant Physiol. 1984 Aug;75(4):993–996. doi: 10.1104/pp.75.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential expression of C4 pathway genes in mesophyll and bundle sheath cells of greening maize leaves. J Biol Chem. 1987 Aug 25;262(24):11726–11730. [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]