Abstract
Background and Aims
The tribe Paullinieae has the highest diversity of vascular variants among the seed plants. The developmental diversity is better understood in the species-rich genera Paullinia and Serjania; however, the phylogeny and diversity of vascular variants in the smaller genera of Paullinieae remain understudied. Here we investigate the evolution of development of stem vasculatures in the small genus Urvillea.
Methods
We generate the first molecular phylogeny of Urvillea derived from 11 markers using a maximum likelihood and Bayesian approach. In combination with phylogenetic reconstruction, stochastic character mapping is used to assess evolutionary changes in stem ontogenies, determined from developmental anatomy of stems collected in the field or from herbarium and wood collections.
Key Results
Urvillea is supported as a monophyletic group and sister to Serjania. There are five stem ontogenies in Urvillea, including typical growth and four different vascular variants. Most stem ontogenies initiate with lobed stems in primary growth. Lobed stems in secondary growth are ancestral in Urvillea, but this ontogeny was lost multiple times. A reversal to typical growth occurred in non-climbing species. Phloem wedges, fissured stems, and ectopic cambia each evolved once independently. Phloem wedges is an intermediate developmental stage in the formation of fissured stems, which is characterized by a continuous fragmentation of vascular tissues. Lobed stems may generate constriction zones and lobes may split or not.
Conclusions
Urvillea is the third most diverse genus (after Serjania and Paullinia) with respect to the number of vascular variants within Paullinieae. One ontogeny (fissured stems) is exclusive to the genus. Differential cambial activity and ectopic cambia are the main ontogenetic processes generating stem diversity. The evolutionary history of vascular variants demonstrates the large developmental plasticity of the cambium in such a small genus and further demonstrates that complex anatomies have repeatedly evolved within Paullinieae lianas.
Keywords: Climbing plants, lianas, ontogeny, phylogeny, fissured stems, stochastic character mapping, vascular variants
INTRODUCTION
In most plants, the vascular cambium is a continuous meristematic region which gives rise to a homogeneous amount of secondary xylem (centripetally) and secondary phloem (centrifugally) across the stem circumference (Spicer and Groover, 2010). This mode of secondary growth has been modified repeatedly throughout vascular plant evolution, generating a massive diversity of secondary growth modes. These variant forms have historically been called ‘anomalous secondary growth’, later termed ‘cambial variants’ and most recently ‘vascular variants’ (Cunha Neto, 2023). This last term recognizes that variant vascular organizations can arise from procambial, cambial and ectopic origins. Following this developmental framework, three categories of vascular variants are recognized (i.e. procambial variants, cambial variants and ectopic cambia), each comprising various anatomical patterns. About 20 patterns are currently recognized in seed plants, each following its own developmental pathway (Cunha Neto, 2023). Vascular variants are interpreted as having noteworthy deviations of the organization, abundance and distribution of vascular tissues compared with the ancestor of seed plants (Hoffman and Tomescu, 2013; Ragni and Greb, 2018; Onyenedum and Pace, 2021). Vascular variants derived from modifications in the procambium (i.e. procambial variants) can be found in families such as Nyctaginaceae with medullary bundles (Cunha Neto et al., 2022) or in Sapindaceae with irregular distribution of vascular bundles in compound stems (Van der Walt et al., 1973; Acevedo-Rodríguez, 1993; Tamaio and Angyalossy, 2009; Lopes et al., 2017). Other types of vascular variants arise from alterations in the activity of a single vascular cambium (i.e. cambial variants), as in the case of phloem wedges in Bignoniaceae or Malpighiaceae (Pace et al., 2009; Quintanar-Castillo and Pace, 2022), while other types derive from the appearance of additional vascular meristems, such as successive cambia in Fabaceae (Leme et al., 2020; Nejapa et al., 2021) or neoformations in Rubiaceae (Leal et al., 2020), all anatomical patterns of ectopic cambia (Cunha Neto, 2023). Whatever the case, these variants generate complex morphologies given the unusual arrangement of vascular tissues, which have been interpreted as ecological adaptations to many different conditions, such as underground reservoir organs, cushion plants, halophytes and especially the climbing habit, as they generate increased hydraulic conduction, flexibility and mechanical resistance (Carlquist, 1991, 2013; Fisher and Ewers, 1991; Rowe et al., 2004). With multiple independent evolutions across gymnosperms and especially angiosperms (Angyalossy et al., 2012, 2015; Cunha Neto, 2023), vascular variants are a compelling system to explore how development is modified to shape plant form during evolutionary time.
The mostly neotropical lineage Paullinieae (Sapindaceae) encompasses six genera (Cardiospermum L., Paullinia L., Serjania Mill., and Urvillea Kunth, Thinuoia Triana & Planch and Lophostigma Radlk.), collectively housing ~475 species of mostly woody climbers (lianas) and occasionally herbaceous vines (Acevedo-Rodríguez et al., 2017). Paullinieae nests the largest diversity of vascular variants among all plant lineages, with about 11 types of variant being currently recognized (Cunha Neto et al., 2018; Chery et al., 2020; Rajput et al., 2021; Rizzieri et al., 2021; Pace et al., 2022). Recent work has focused on characterizing the developmental anatomy of patterns called ‘compound vascular cylinder’ (Tamaio and Angyalossy, 2009), ‘corded vascular cylinder’ (Tamaio and Somner, 2010), ‘divided vascular cylinder’ (Araújo and Costa, 2006; Rizzieri et al., 2021), ‘successive cambia’ and ‘neoformations’ (Bastos et al., 2016; Cunha Neto et al., 2018), ‘lobed stem’ (Chery et al., 2020) and ‘phloem wedges’ (Pellissari et al., 2018; Chery et al., 2020; Rajput et al., 2021). When this diversity is explored in a phylogenetic context, the species-rich genera Serjania and Paullinia stand out as the most diverse in terms of number of vascular variants (Bastos et al., 2016; Cunha Neto et al., 2018; Pace et al., 2022). However, the phylogeny and diversity of vascular variants in the smaller genera of Paullinieae remain understudied, particularly Urvillea which is often cited to have lobed stems and fissured stem (= fissured vascular cylinder, following Tamaio et al., 2011).
Urvillea encompasses 20 species of lianas, vines and rhizomatous hemicryptophytes that grow mostly in tropical forest of the Americas (Fig. 1), extending from Texas (USA) to Argentina and Uruguay (Ferrucci, 2020). Stem anatomy of these plants has been described mostly using Urvillea rufescens as a reference, which is generally described as having lobed or fissured stems (see glossary of terms in Table 1) (Tamaio et al., 2011; Bastos et al., 2016; Pace et al., 2022). In general, Urvillea species have a lobed stem outline and if the stem fragments or breaks apart into lobes or separate units, they are considered fissured stems (Tamaio et al., 2011; Bastos et al., 2016). Nevertheless, other reports indicate that distinct stem developments may also occur. Radlkofer (1931) and Metcalfe and Chalk (1950) illustrated the stem of U. laevis as having some type of ‘dissected’ vascular cylinder, called ‘corpus lignosum fissum’ (Radlkofer, 1931) or ‘clef xylem mass’ (Metcalfe and Chalk, 1950). The dissected portions of vascular tissue were named ‘vascular cylinders’ by Lopes et al. (2017) and Ferrucci (2020) by studying the same species. Based on these studies, which characterize the developmental anatomy, we hypothesize that these units do not constitute true ‘vascular cylinders’, and thus are not homologous to the peripheral cylinders observed in other variants, e.g. compound stems. In compound stems, vascular cylinders derive from atypical organization of vascular bundles (i.e. procambial variant), which form multiple cambia that generate the cylinders (Cunha Neto, 2023). However, ontogenetic studies are still lacking for most Urvillea species. In addition, the presence of ‘supernumerary cambia’ was reported for U. filipes, but neither anatomical nor developmental studies were performed (Ferrucci, 2020). Moreover, unlike other genera that encompass mostly woody vines, Urvillea also includes herbaceous plants with rhizomatous hemicryptophyte decumbent habit (e.g. U. procumbens and U. pterocarpa; Ferrucci, 2020). Thus, it is unknown whether vascular variants in Urvillea relate to the climbing habit or not, a correlation that was validated in some lineages (Quintanar-Castillo and Pace, 2022), but not all (Cunha Neto et al., 2022).
Fig. 1.
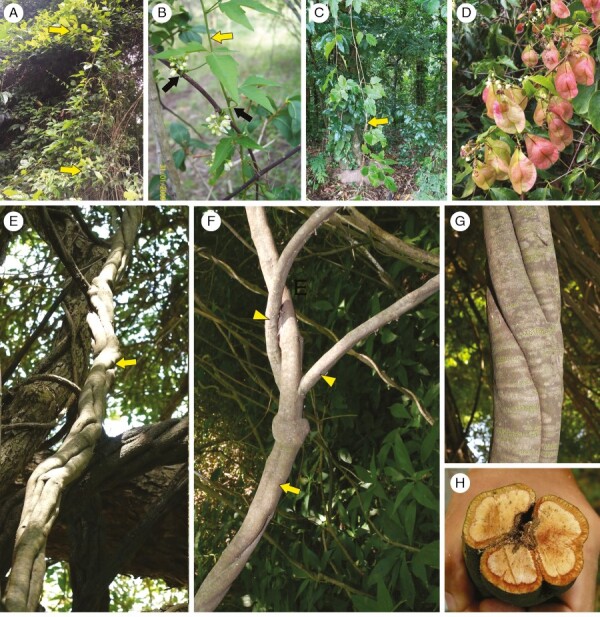
Urvillea plants in the field. (A) Urvillea stipularis: woody vine growing in Atlantic Forest. Bahia, Brazil. (B) Urvillea uniloba: young shoots (yellow arrow) with tendrils (black arrows). Buenos Aires, Argentina. (C) Urvillea stipitata: branches of woody vine (yellow arrow) growing on campus of the Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil. (D) Urvillea stipularis: fruits of plant growing in Atlantic Forest. Espírito Santo, Brazil. Image courtesy of Geovane S. Siqueira. (E–H) Urvillea uniloba. Buenos Aires, Argentina. (E) Large woody stem (yellow arrow) climbing up a host tree. (F) Main stem (yellow arrow) and bifurcations (yellow arrowheads) likely due to stem splitting and/or fragmentation. (G) External morphology showing tortuous stem. (H) Cross-section of mature stem undergoing fragmentation into three portions (stem splitting).
Table 1.
Glossary of terms.
| Term | Definition |
|---|---|
| Ectopic cambia | Vascular development where de novo cambia arise from dedifferentiation/redifferentiation of parenchyma cells generating multiple cambia nested in the same organ; a category of vascular variants (Cunha Neto, 2023). |
| Fissured vascular cylinder | Also called fissured stem; describes instances where differential cambial activity generates phloem wedges followed by disruption of the cambium continuity and dissection of vascular tissues; a type of cambial variant. |
| Phloem wedges | Defined as sectors of the cambium with increased production of secondary phloem and decreased amount of secondary xylem; a type of cambial variant. |
| Stem splitting | Describes the entire woody stem separating longitudinally into individual units, each unit comprising pith, secondary xylem, cambium, secondary phloem and periderm. |
| Typical growth | Stem development derived from a typical eustele and a single, bifacial cambium generating vascular tissue at homogeneous rates. |
In this study we investigate the developmental anatomy of Urvillea stems to determine their structural diversity and systematic significance. Furthermore, we explore the ontogenetic data within a phylogenetic framework through the first molecular phylogeny for the genus to elucidate how modifications to developmental trajectories shaped the diversity of vascular variants during the evolution of the genus.
MATERIALS AND METHODS
Sampling for anatomy
We sampled 14 species (23 specimens) of Urvillea for anatomical and developmental studies (Table 2). In the field, samples were collected at different developmental stages, including stems near the apex and along the stem, including fully developed stems at the base of the plants. Samples were immediately fixed in FAA50 (formaldehyde:acetic acid:alcohol; 1:1:18) and then stored in 70 % ethanol (Johansen, 1940). Some specimens were obtained from herbarium vouchers and processed as indicated below. Whenever possible more than one specimen was investigated for each species. In all species, we analysed the mature stems to capture the final stem morphology, but full developmental studies including multiple stages were used for some species (Table 2).
Table 2.
Studied Urvillea species including information on species name, collection site, collector and herbarium data, type of sample and stem diameter. The column ‘Sample’ indicates whether the specimen was used for anatomy (stem) and/or phylogenetic studies (DNA). Asterisks (*) indicate specimens used for developmental study.
| Species | Collection site | Collector, collector number (herbarium, voucher) | Sample | Stem diameter1 (mm) |
|---|---|---|---|---|
| Urvillea andersonii Ferrucci | Caetité, Bahia, Brazil | Arbo, 7639 (US 3390103) | Stem | 10 |
| Bom Jesus da Lapa, Bahia, Brazil | Harley, 21549 (US 3182658) | DNA | NA | |
| Urvillea chacoensis Hunz. | Botanic Garden of Departamento Santa Cruz, Bolivia | Acevedo-Rodríguez, 4641 (US 3630532) | Stem | 9 |
| Ñuflo de Chavez, Santa Cruz, Bolivia | Ferrucci, 3138 (US 01257434) | DNA | NA | |
| Provincia Florida, Santa Cruz, Bolivia | Nee, 45185 (US 00734822) | DNA | NA | |
| Urvillea filipes Radlk. | Germán Busch, Santa Cruz, Bolivia | Ferrucci, 3131 (US 01257440)2 | DNA | NA |
| Warnes, Santa Cruz, Bolivia | Nee, 45143 (US 3442958) | Stem | 4 | |
| Urvillea glabra Cambess. | Parque Natural Municipal de Grumari, Rio de Janeiro, RJ, Brazil | Somner, 1624 (RBR 44403)* | Stem, DNA | 19 |
| Urvillea intermedia Radlk. | Montes Claros, Minas Gerais, Brazil | Carvalho, 6585 (US 00735001) | DNA | NA |
| São Felipe, Bahia, Brazil | Lordêlo, 56-480 (ALCB 06510)2 | Stem | 9 | |
| Urvillea laevis Radlk. | Vale Santa Maria do Rio Doce, Espírito Santo, Brazil | Acevedo-Rodríguez, 3719 (US 3183996)* | Stem | 17 |
| Corumbá, Mato Grosso do Sul, Brazil | Acevedo-Rodríguez, 16762 (US 3734193) | Stem, DNA | 20 | |
| Sapeaçu, Bahia, Brazil | Lacerda, 2602 (HURB 31604) | Stem | 12 | |
| Urvillea oliveirae (Ferrucci) Acev.-Rodr. & Ferrucci | Pau-Ferro, Ipirá, Bahia, Brazil | Guedes, 21171 (ALCB 113061)2 | Stem | 7 |
| Urvillea pterocarpa (Radlk.) Acev.-Rodr. & Ferrucci | Sidrolândia, Mato Grosso do Sul, Brazil | Urdampilleta, 321 (US 3563492) | Stem | 4 |
| Urvillea rufescens Cambess. | Restinga de Grumarí, RJ, Brazil | Somner, 1615 (RB 00728176)* | Stem | 20 |
| Reserva Biológica Poço das Antas, Rio de Janeiro, RJ, Brazil | Somner, 1637 (RBR 58244) | Stem | 14 | |
| Reserva Biológica Poço das Antas, Rio de Janeiro, RJ, Brazil | Somner, 1644 (RBR 58245) | Stem | 10 | |
| Urvillea stipitata Radlk. | Parque Nacional do Itatiaia, Rio de Janeiro, Brazil | Somner, 1448 (RB 00633824)* | Stem, DNA | 18 |
| Campus da UFRRJ, Seropédica, RJ, Brazil | Santos & Somner, 53 (RBR 56155) | DNA | NA | |
| Urvillea stipularis Ferrucci | Reserva Valle do Rio Doce, Linhares, Espírito Santo, Brazil | Acevedo-Rodríguez, 3695* (US 3233686) | Stem, DNA | 17 |
| Mata de Cazuzinha, Cruz das Almas, Bahia | Martins, 2339 (HURB 26840)* | Stem | 15 | |
| Urvillea triphylla (Vell.) Radlk. | Reserva Valle do Rio Doce, Linhares, Espírito Santo, Brazil | Acevedo-Rodríguez, 3711 (US 3212571)* | Stem | 8 |
| Urvillea ulmacea Kunth | Reserva Valle do Rio Doce, Linhares, Espírito Santo, Brazil | Acevedo-Rodríguez, 3688 | Stem | 30 |
| Parque Nacional Palo Verde Guanacaste, Costa Rica | Chavarria, 386 (US 3300682) | Stem | 15 | |
| Bonito, Mato Grosso do Sul, Brazil | Acevedo-Rodríguez, 16498 (US 3734040) | Stem | 10 | |
| Chaa Creek, Belize | Balik 3349 (MO 05072347) | DNA | NA | |
| Urvillea uniloba Radlk. | Corumbá, Mato Grosso do Sul, Brazil | Acevedo-Rodríguez, 16762 (US 3734193) | Stem | 25 |
| Santo Tomé, Corrientes, Argentina | Arbo, 5816 (US 00735204) | DNA | NA | |
| Isla Martín García, Buenos Aires, Argentina | Cabanillas, 6, 146 (LP)* | Stem | 50 | |
| Isla Martín García, Buenos Aires, Argentina | Cabanillas, 147 (LP) | Stem | 45 |
1Largest stem diameter investigated.
2Anatomical studies were based on samples from vouchers.
Procedures for light microscopy
We used similar protocols to process samples for anatomical studies, which were performed in different laboratories at the following institutions: Museu Nacional, Universidade Federal do Rio de Janeiro (UFRJ); Onyenedum Lab, Cornell University (CU); Cátedra de Dendrología, Universidad Nacional de La Plata (UNLP); and Universidade Federal do Recôncavo da Bahia (UFRB). Samples of several species were initially hand-sectioned using a razor blade to detect the main developmental stages. These sections were stained with Safrablau (a 9:1 mixture of 1 % Astra Blue in 50 % ethanol and 1 % Safranin O in 50 % ethanol) and mounted in 50 % glycerol to make semi-permanent slides (Kraus and Arduin, 1997). Specimens from herbarium vouchers were rehydrated by boiling the samples until they sank. These samples were processed in (2-hydroxyethyl)-methacrylate (Historesin Embedding Kit, Leica, Heidelberg, Germany). Samples containing the selected stages were processed following different methods. Young small samples (1–5 mm) were embedded in historesin, sectioned at ~3–12 µm using a rotary microtome at CU (Olympus CUT 4060) or UFRB (Leica RM2245), and stained with toluidine blue (O’Brien et al., 1964). Larger stem samples were embedded in polyethylene glycol 1500, sectioned at variable thickness (~16–25 µm) with a sliding microtome (Leica SM2000R at UFRJ; Spencer 880 at CU; Reichert OmE at UNLP) or a xylotome at UNLP (Sledge Microtome Jung) following Barbosa et al. (2010). Sections were stained with Safrablau or double stained with safranin 1 % in 80 % alcohol, and fast green 1 % in alcohol 100 % at UNLP. Sections were mounted in synthetic resins (e.g. Permount resin, Fisher Scientific, Pittsburgh, PA, USA) to make permanent slides.
The slides were examined and documented using different imaging setups where samples were produced as follows: Leica DM750 light microscope equipped with a Leica ICC50 digital camera at UFRJ; Ernst Leitz Wetzlar WT3 light microscope equipped with an Olympus X-940 digital camera at UNLP; and an Olympus BH2 with an Amscope MU1000 digital camera at CU.
Figure 6B was generated from a fixed sample of Urvillea chacoensis using Laser Ablation Tomography (LATscan) following Cunha Neto et al. (2023).
Fig. 6.
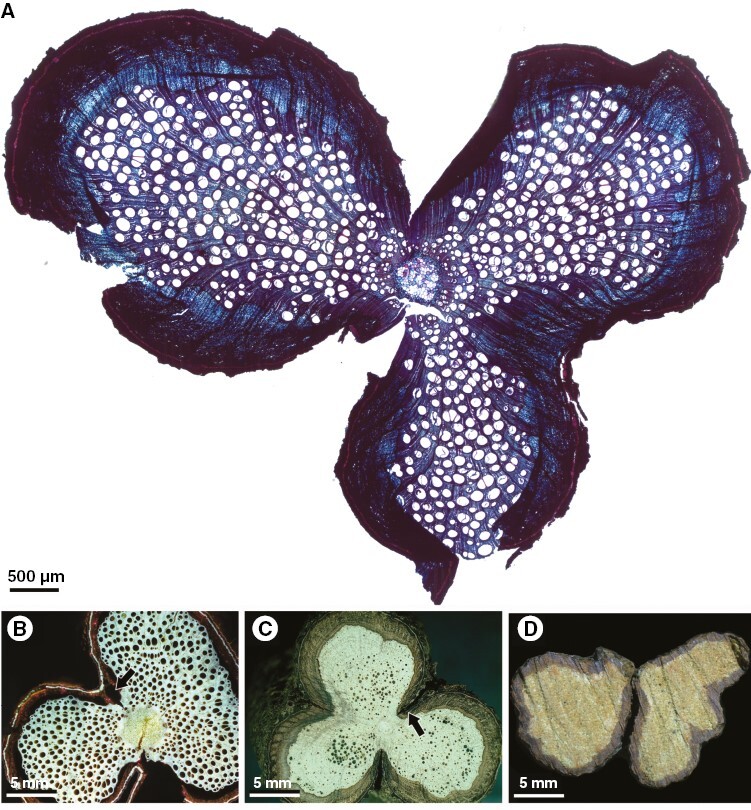
Cross-sections of Urvillea species showing lobed stems without and with stem splitting (ontogeny 4). (A) Urvillea stipitata, three-lobed stem (light microscopy); stem splitting is expected in the furrow region. (B) Urvillea chacoensis, two-lobed stem (laser ablation tomography, LATscan); stem splitting is expected in the furrows, forming a constriction zone (arrow). (C) Urvillea rufescens, three-lobed stem; stem splitting is expected in the furrows forming a constriction zone (arrow). (D) Urvillea stipitata, split stem; the two parts are detached and were grouped to image the stem.
Procedures for macroscopic analyses
Mature stems were generally polished by manually grinding the surface in waterproof sandpapers under water following Barbosa et al. (2021). Images were taken using different setups where samples were produced as follows: Canon Eos Rebel Xti camera at UNLP or Nikon SMV1500 stereoscope (Tokyo, Japan) with a Nikon Digital Sights Fi-3 camera running Nikon Elements F software (version 4.60) at the Specht Lab (Cornell University). Additional images were obtained from the online database Lianas and Climbing Plants of the Neotropics (Acevedo-Rodríguez, 2015 onwards; reproduced with permission).
Molecular dataset and phylogenetic analysis
The phylogeny consisted of 15 species of Urvillea and outgroups, including nine species of other genera of Paullinieae and Allophylus psilospermus Radlk., a member of the Thouiniaeae tribe (sensuAcevedo-Rodríguez et al., 2017), which is used to root the tree. Species names, voucher information, markers and GenBank accession numbers for all sequences are provided in Supplementary Data Table S1. Some sequences were generated for this study and other sequences were derived from previous phylogenetic studies (Acevedo-Rodríguez et al., 2017; Chery et al., 2019), which were downloaded from GenBank (Supplementary Data Table S1). The final dataset consisted of 32 accessions from 25 species and 11 molecular markers, including plastid trnL intron (regions c and d, from Taberlet et al., 1991) and psbA-trnH (from Demesure et al., 1995 and Fazekas et al., 2008), and the nuclear internal transcribed spacer (ITS4-5 from White et al., 1990) and nine single-copy nuclear markers following Chery et al. (2017, 2019).
DNA extraction, amplification and sequencing.
DNA extractions were performed using the CTAB method (Doyle and Doyle, 1987). The quality and quantity of total DNA extracts were evaluated using a Nanodrop and Qubit Fluorometer (Life Technologies, Carlsbad, CA, USA). The extracts were analysed on a 1 % agarose gel to determine the size of the fragments. Amplification of selected regions was achieved in 10- or 20-μL reaction mixtures containing 2 μL of 5X Phire buffer (contains 7.5 mm MgCl2), 0.2 μL 10 mm dNTPs, 0.5 μL 10 μm primers, 0.1 μL Phire Hot Start II DNA Polymerase (Thermo Scientific, Waltham, MA, USA), 3 μL template DNA and 3.7 μL ddH2O. Volumes were doubled for 20-μL reactions. PCR was performed in a Bio-Rad thermos cycler (S1000). The following PCR program was used for all primers: initial denaturation was programmed for 5 min at 98°C, followed by 35 cycles at 98°C for 5 s, 60°C for 5 s and 72°C for 20 s, plus a final extension of 1 min at 72°C. PCR products were run in 1 % agarose gel to confirm amplification and were later purified using ExoSap following the manufacturer’s protocol for sequencing. DNA extractions and amplifications were performed at the Onyenedum Lab (Cornell University). Sanger sequencing was performed with the same primers used for the PCR amplifications, using BigDye Terminator Cycle Sequencing and a 3730xl Genomic Analyzer at Cornell Biotechnology Genomics.
Alignment and phylogenetic analysis.
Novel sequences were trimmed using Geneious v.8.0.5 (Biomatters, Auckland, New Zealand) and aligned with sequences downloaded from GenBank. For each gene, sequences were aligned using MUSCLE with the default parameters as implemented in Geneious v.8.0.5. Aligned sequences for each gene were concatenated using Geneious and visualized using AliView v.1.28 (Larsson, 2014). The final concatenated alignment was used for phylogenetic inference using maximum likelihood (ML) and Bayesian inference. The best-fit partitioning scheme and models of evolution were obtained from the ModelFinder feature in IQ-TREE on the CIPRES Science Gateway portal (Miller et al., 2010), then applied for ML tree inference. For ML analysis, we used IQ-TREE v.2.1.2 and performed bootstrapping with 1000 replicates. For the Bayesian inference partitioned analysis, we used a modified set of substitution models (based on the ModelFinder Result) that can be accommodated by MrBayes v.3.2.7. The Monte Carlo Markov chain was run with two runs each of four chains, for 10 million generations with 25 % (or 2.5 million) burn-in and trees sampled every 1000 generations. We considered the convergence of Markovian chains using the log posterior probability and if the effective sample size was ≥200 as analysed by Tracer v.1.7.2 (Rambaut et al., 2014). We combined the posterior probability of trees using the text editor BBEdit v.14.6.3 (Bare Bones Software; http://www.barebones.com/). Next, we used TreeAnnotator v.1.10.4 (BEAST v.1.10 package; Suchard et al., 2018) to generate the maximum clade credibility (MCC) tree using the post-burn-in trees from the combined MrBayes runs (excluding 2.5 M burn-in trees from each run), with median node heights. The resulting tree and node-specific population size was visualized in FigTree v.1.4.4.
Character coding and phylogenetic comparative methods
To understand the evolution of vascular anatomies in Urvillea we first determined distinct ontogenetic trajectories based on developmental studies as indicated earlier. To reconstruct the evolution of stem ontogenies, we coded each ontogenetic trajectory found in Urvillea and outgroup species as a distinct character state (e.g. ontogeny 1 = 1, ontogeny 2 = 2 etc.). Ontogenies are described in the Results section (Fig. 2). The MCC tree generated previously was pruned to those Urvillea species with stem ontogeny data, using the drop.tip function in the phytools R package (Revell, 2012). For ancestral state reconstructions, we excluded species without anatomical data (e.g. U. procumbens) or those whose anatomical data were obtained only from small stems from herbarium specimens with uncertain stem ontogeny (e.g. U. oliveirae, U. intermedia). The final dataset comprised 11 Urvillea species and nine outgroup species. Ancestral states were estimated and visualized using stochastic character mapping with the make.simmap function (Revell, 2013), under the best fit-model of evolution (ER) determined by the Akaike information criterion (Supplementary Data Table S2) in the function fitDiscrete of the R package geiger (Pennell et al., 2014). One thousand character histories were simulated along the maximum clade credibility tree to account for the different character histories and the results were summarized with the plot_simmap function written by Dr Michael May (University of California, Berkeley, USA). All analyses were performed in R (R Core Team, 2022). The sequence alignment, phylogenies and codes are available at 10.5281/zenodo.7754041.
Fig. 2.
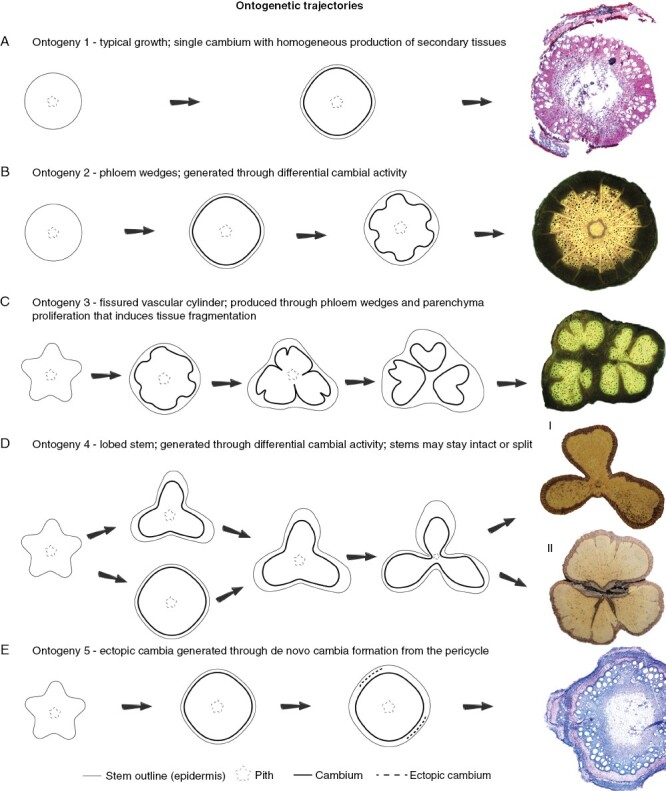
Schemes of ontogenetic trajectories in Urvillea. Far right images indicate most developed stems in microscopic (A, E) or macroscopic (B–D) images. (A) Ontogeny 1 is represented here by U. pterocarpa, which is characterized by the typical secondary growth observed in prostrate and rhizomatous species. (B) Ontogeny 2 is exclusive to U. stipularis, in which phloem wedges are regularly distributed and delimited by limiting rays. (C) Ontogeny 3 is exclusive to U. laevis, which has the vascular system fragmented multiple times due to phloem wedges that segment the secondary phloem, secondary xylem and pith. (D) Ontogeny 4 is represented by lobed stems, which is the most common ontogeny. In this type, stems undergo differential cambial activity in sectors of the cambium-generating lobes (more vascular tissue) and furrows (less vascular tissue). In the final developmental stage, this type is represented by stems without [U. ulmacea (I)] or with [U. uniloba (II)] stem splitting. Stem splitting results from continued differential activity of the cambium producing less vascular tissues in the furrows and more tissue in the lobes. Differential activity through phloem wedges also contributes to this process. (E) Ontogeny 5 is exclusive to U. filipes, and is the only one with de novo formation of cambium, characterizing the presence of ectopic cambia.
RESULTS
We found five ontogenies in Urvillea, one with typical growth and four types of vascular variants. Stem ontogenies initiate development with lobed or seemingly circular outline (Fig. 2). The primary vasculature is formed by a eustele with multiple vascular bundles delimiting the pith (Figs 3A and 5A). In all species, the cambium initiates in the typical way producing secondary xylem to the inner side and secondary phloem to the outer side. However, differences in cambial activity determine different stem ontogenies (summarized in Fig. 2). Below we describe the developmental trajectory of each stem ontogeny, with emphasis on anatomical modifications and developmental processes.
Fig. 3.
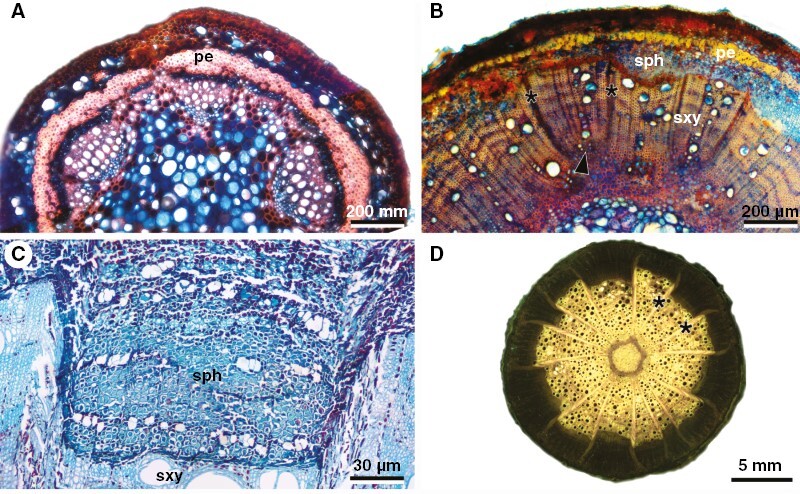
Developmental anatomy of U. stipularis stems (ontogeny 2). (A) Lobed young stem showing a typical cylinder development. (B) Early stages of phloem wedges in young stems. Note the irregular production of xylem and phloem in the centre; note also developing limiting rays (asterisks), which are formed from the cambium in interfascicular regions [notice that the primary xylem (arrowhead) is located between the two rays]. (C) Detail of a phloem wedge delimited by limiting rays (see panel D). (D) Macroscopy of the whole stem showing the organization of phloem wedges separated by delimiting rays (asterisks). pe, pericycle; sph, secondary phloem; sxy, secondary xylem.
Fig. 5.
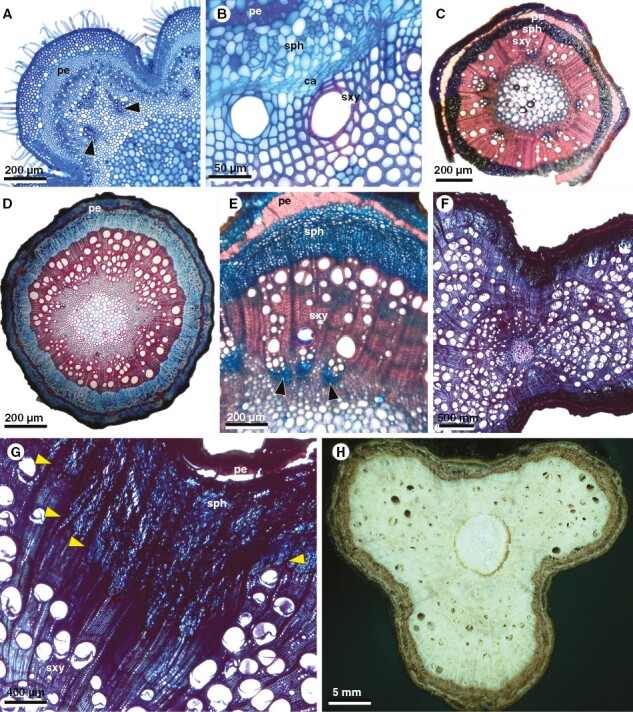
Developmental stages in stems of Urvillea species with ontogeny 4. Stems generally begin with a lobed stem, undergo a stage of nearly round circular outline, and then change to a permanent lobed structure, due to the decreased production of vascular tissues in the furrows and increased formation of vascular tissues in the lobes. (A–C) Urvillea ulmacea. (A) Young stem with lobed stem outline in primary growth; note poles of protoxylem (arrowheads) of vascular bundles. (B) Detail of cambium establishment. (C) Nearly circular stem outline after some period of secondary growth with homogeneous rates of vascular tissue production. Images A and B courtesy of William A. L. Lopes. (D) Urvillea uniloba; early secondary growth showing homogeneous rates of vascular tissues and circular stem outline. (E) Urvillea chacoensis; initial stages of differential cambial activity due to more secondary xylem and phloem being produced in the centre of the lobe, indicated by the presence of vascular bundles with poles of protoxylem (arrowheads). (F–H) Urvillea rufescens. (F) Two-lobed mature stem. Note that in the furrows almost no vessels are produced with the xylem formed mostly by fibres. (G) Detail of image in (F) showing increased secondary phloem production in relation to secondary xylem delimited by the rays (arrowheads). (H) Three-lobed mature stem (macroscopy). ca, cambium; pe, pericycle; ra, vascular ray; sph, secondary phloem; sxy, secondary xylem.
Ontogeny 1, typical growth: single cambium with typical activity producing a limited amount of secondary tissues (Fig. 2A)
In this ontogeny, the vascular cambium is formed in the usual way for eudicots with typical growth (Fig. 2A). The cambium maintains the typical activity throughout stem development but produces a limited amount of secondary xylem and secondary phloem (Fig. 2A). Adult stems maintain the cylindrical stem shape (Fig. 2A). This pattern is observed in the aerial shoots of the rhizomatous species U. pterocarpa (Fig. 2A).
Ontogeny 2, phloem wedges: single cambium with differential cambial activity generates phloem wedges regularly distributed; cambium is continuous and limiting rays are present (Figs 2B and 3A–D)
This ontogeny is exclusive of Urvillea stipularis (Fig. 2A). Stem ontogeny initiates with circular stem outlines (Fig. 3A). As secondary growth begins, differential cambial activity is observed between the interfascicular and fascicular cambium. The interfascicular cambium generally produces mostly rays, which become relatively wide as stem development progresses (Fig. 3B, C). The fascicular cambium produces secondary vascular tissues in the usual polarity (Fig. 3B). Nevertheless, since early secondary growth (stems ~1–2 mm wide), differential cambial activity leads first to phloem arcs. As secondary growth progresses, these phloem arcs convert into phloem wedges due to the continued differential activity of the cambium in these same discrete regions (Fig. 3C). Fully developed stems have a circular stem outline with several phloem wedges delimited by wide limiting rays (Fig. 3C, D). The cambium remains continuous throughout the stem circumference (Fig. 3C).
Ontogeny 3, fissured vascular cylinder: single cambium with differential cambial activity generates phloem wedges irregularly distributed; the cambium is discontinuous, and the vascular system is compartmentalized; limiting rays are absent (Figs 2C and 4A–C)
Fig. 4.
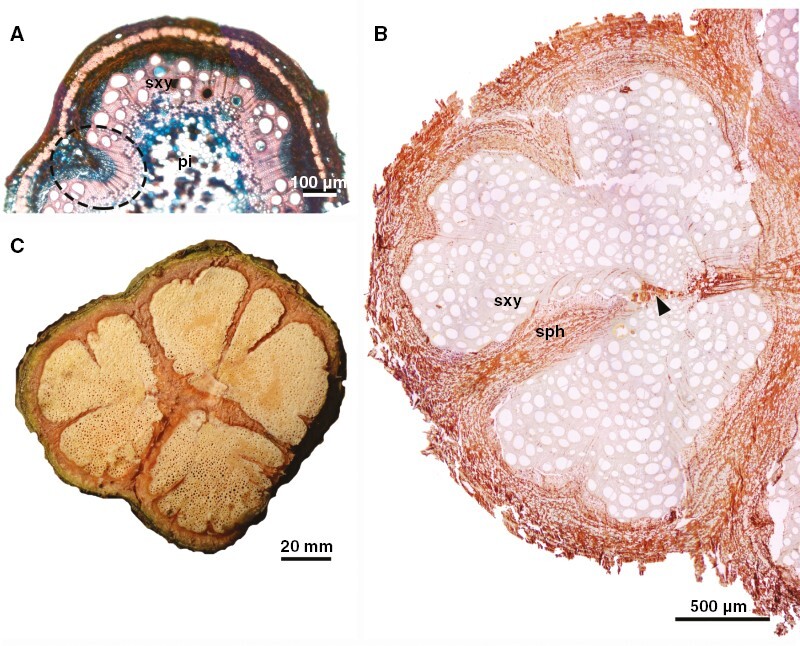
Developmental anatomy of stems of U. laevis (ontogeny 3). (A) Early stage of secondary growth showing premature formation of phloem wedges (ellipse). (B) Light microscopy (unstained) of developmental stage presented in (C), showing different sizes of phloem wedges dissecting the vascular system. Note also disruptive parenchyma (arrowhead), which also contributes to vascular tissue dissection. (C) Macroscopy of stem showing late developmental stages where furrows have fissured the vascular cylinder. sph, secondary phloem; sxy, secondary xylem; pi, pith.
This ontogeny is exclusive to U. laevis (Fig. 2C). Stems are relatively lobed during primary growth (Figs 2A and 4A). The vascular cambium arises in the usual manner, connects all vascular bundles distributed across the stem circumference, and produces secondary vascular tissues in the usual polarity (Fig. 4A). Some sectors of the cambium display differential cambial activity producing phloem arcs and/or phloem wedges from early secondary growth (stems ~1–2 mm wide; Fig. 4A). In some phloem wedges, cambial anticlinal division ceases in the limits of the phloem wedges, which makes the cambium discontinuous, segmenting the vascular tissues (Fig. 4B). After disruption of the cambial ring, each cambial segment continues to divide and produce vascular tissues forming nearly circular compartmentalized units of vascular tissues (Fig. 4B, C). The vascular system becomes compartmentalized in at least two or three major portions, segmenting the stem from the secondary phloem through the pith (Fig. 4B). Parenchyma proliferation is also present and contributes to vascular tissue dissection (Fig. 4B). As secondary growth progresses, additional phloem wedges and invaginations further dissect the previous compartmentalized regions into new smaller portions (Fig. 4B, C). Mature stems may have multiple compartments, but the stem circumference remains nearly circular (Fig. 4C). Longitudinal stem splitting was not observed.
Ontogeny 4, lobed stems: single cambium with differential activity generates lobes and furrows with or without stem splitting (Figs 2D, 5A–H, 6A–D and 7A–D)
Fig. 7.
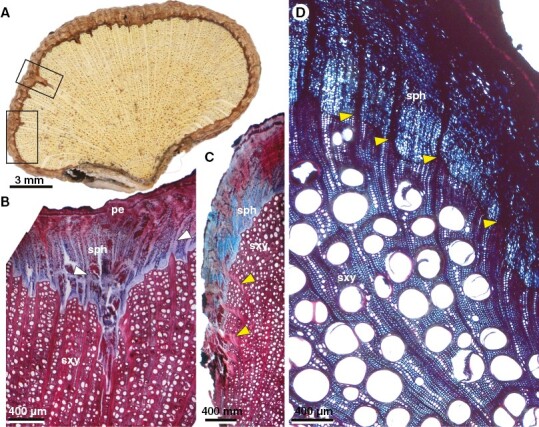
Details of lobed stems and stem splitting (ontogeny 4). (A–C) Urvillea uniloba. (D) Urvillea stipitata. (A) Split lobe. Note the formation of phloem wedges (top box; see detail in panel B) and rays with curved path in the periphery of the lobe (bottom box; detail in panel C). (B) Detail of phloem wedge and large rays (white arrowheads) with straight course near the phloem wedge. (C) Curved rays in the secondary xylem (yellow arrowheads) in the lateral part of the lobe. (D) Curved rays running from secondary xylem to secondary phloem (yellow arrowheads) in the lateral part of the lobe. pe, pericycle; sph, secondary phloem; sxy, secondary xylem.
Stem ontogeny usually initiates with a lobed outline, with vascular bundles organized along the lobes (Fig. 5A). The vascular cambium is established and produces secondary xylem and secondary phloem in regular rates for some time (Fig. 5B), generating a stem with a circular outline (Fig. 5C–D). Next, differential activity by the cambium (Fig. 5E) represses the homogeneous rates of secondary vascular tissue at approximately equidistant sites along the stem circumference; relative to the furrows, the lobes experience increased production of secondary xylem and secondary phloem (Fig. 5F). In the repressed secondary growth of the furrows, the xylem is mostly composed of fibres and there are relatively few vessels compared with the lobes (Fig. 5F). In U. uniloba the furrows present a slight increase in phloem production/phloem expansion, compensating part of the lobed structure (Fig. 8). Despite the lobed outline of the stem, the cambium remains continuous throughout all developmental stages (Fig. 5G). With continued secondary growth, the difference between lobes and furrows becomes increasingly pronounced, and some furrows become the site of constriction zones near the pith (Fig. 6A–C).
Fig. 8.
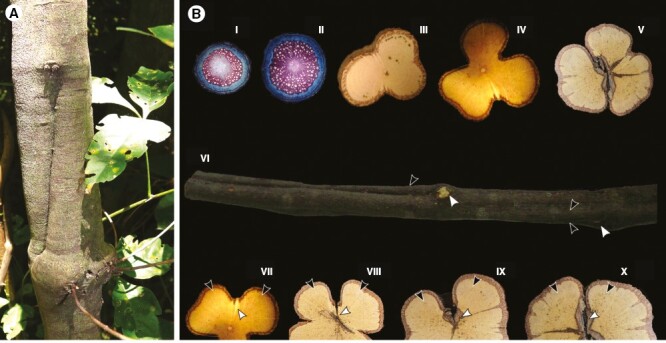
Relation between stem morphology, anatomy and leaf orthostichies in U. uniloba. (A) Stem in the field showing lobes and furrows, and nodes in front of the furrows. (B) Schematic image showing relation between lobed stems and orthostichies. (I, II) Stem in early developmental stages with nearly homogeneous rates of vascular tissue (~2–5 mm in diameter) (III, IV) Lobed stems (~12–22 mm in diameter). (V) Split stem with phloem wedges in the lobes (~22 mm in diameter). (VI) Stem ~22 mm in diameter. Note that lobes split (black arrowhead) under the node (white arrowhead). (VII–X) Note that the furrows (white arrowheads) are the weakness zone between lobes (black arrowheads), where the stem eventually splits into different portions.
Adult stems have either two-lobed configuration, as in U. chacoensis (Fig. 6B) or 3-lobed stems as in U. stipitata (Fig. 6A), U. rufescens (Fig. 6C), or U. ulmacea (Fig. 2D). In some species (e.g. U. ulmacea, U. chacoensis and U. triphylla) the two- or three-lobed configuration is the final developmental stage (without stem splitting) (Fig. 6C). In other species the stems break apart in the constriction zones, as in U.stipitata (Fig. 6D) and U. rufescens (Fig. 2D), separating one or more lobes, which may continue to grow as an independent stem unit as in U. uniloba (Fig. 1F, H). In the split lobes, vascular rays remain straight in the central portion (Fig. 7A, B), whereas dilated rays tend to bend towards the lateral region (previously the furrows) (Fig. 7A, C, D). Small phloem wedges are also observed in mature stems across the stem circumference (Fig. 7A).
Lobed stems and phloem wedges in relation to leaf orthostichy: a case study in U. uniloba (Fig. 8A, B).
The position of furrows and phloem wedges are generally below leaf orthostichies, which have alternate phyllotaxy. The alternation between lobes and furrows generates a tortuous stem, which is observed from the external morphology (Fig. 8A, B). In mature stems, lobes and furrows are maintained because the cambium continues to produce increased rates of vascular tissues in the lobes, but greatly decreases the production of xylem in the furrows (Fig. 8B), which is the region associated with stem splitting (Fig. 8B).
Ontogeny 5, ectopic cambia: de novo cambia developing from procambial derived cells (Figs 2E and 9A–D)
Fig. 9.
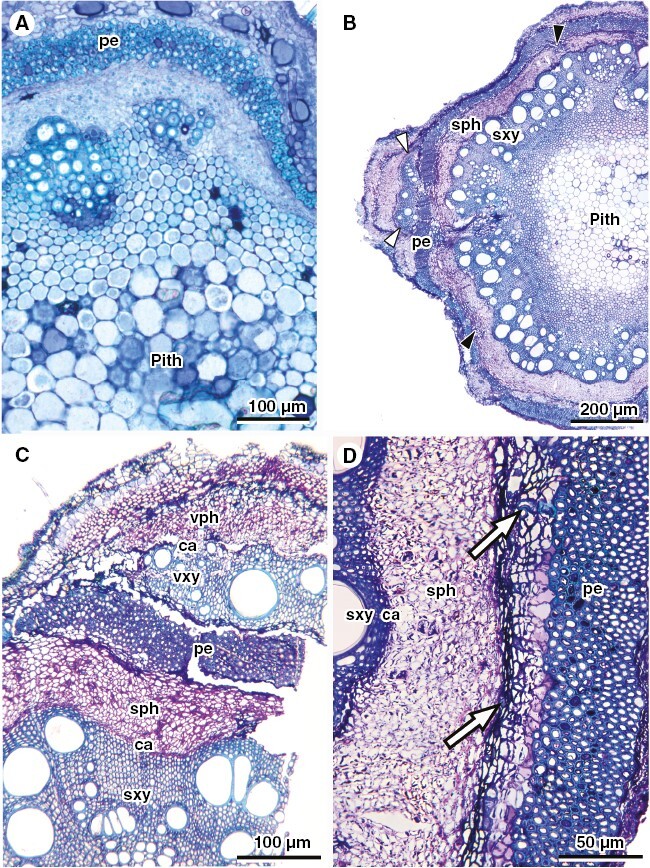
Stem anatomy of U. filipes, representing ontogeny 5. (A) Primary growth showing typical early development of lobed stems. Image courtesy of Maria Camila Medina. (B) General view of stem with ectopic cambia located outside the fibrous pericycle (white arrowheads) and internally to the pericycle (black arrowheads). (C) Detail of the ectopic cambium outside the pericycle, producing variant xylem and variant phloem in the usual polarity. (D) Detail of differentiation of a new vascular cambium internally to the fibrous pericycle, where white arrows point to lignified cells derived from this new cambium. ca, cambium; sph, secondary phloem; sxy, secondary xylem; pe, fibrous pericycle.
Urvillea filipes stem development is similar to ontogeny 1, except that the stem outline is lobed at early ontogenetic stage (Fig. 9A). Secondary growth is established in the typical usual way, but later new vascular cambia arise from parenchyma located outwards (Fig. 9B-C) and inwards (Fig. 9B, D) to the fibrous pericycle. New cambia produce secondary xylem (centripetally) and phloem (centrifugally) in their discrete location (Fig. 9C). None of the new cambia are continuous.
Molecular phylogeny and ancestral state reconstruction
The combined dataset includes 32 terminals and 5200 bp. The least informative gene was the trnL intron (pairwise identity 98 %) and most informative were Orange1.1g002083 (pairwise identity 71.9 %) followed by the ITS (pairwise identity 74.1 %) (Supplementary Data Table S1). The ModelFinder best scheme favoured eight models corresponding to the 11 partitions (Supplementary Data Table S1). The sequence alignment and phylogeny (Supplementary Data Dataset S1) are available at https://zenodo.org/deposit/7754041.
The ML and the Bayesian 50 % majority-rule consensus tree are well resolved, with most nodes having >75 bootstrap values or >0.95 posterior probability (Fig. 10), respectively, except for the placement of U. andersonii + U. laevis, which is unresolved (Fig. 10). The posterior probabilities between the two accessions of U. pterocarpa and U. procumbens are low and the branch lengths are nearing 0 in both the ML and the MCC tree, thus indicating their very close affinity (Supplementary Data Figs S1 and S2). Within Paullinieae, Thinouia is sister to the rest of the genera comprising Paullinieae, followed by Lophostigma, which is followed by Paullinia and a Urvillea + Serjania clade (Fig. 10). Urvillea species are grouped in three main clades. Clade 1 comprises four species: U. andersonii, U. stipularis, U. laevis and U. stipitata; clade 2 includes four species: U. oliveirae, U. filipes, U. procumbens, and U. pterocarpa; clade 3 comprises seven species: U. cuchujaquensis, U. glabra, U. ulmacea, U. intermedia, U. rufescens, U. uniloba and U. chacoensis. Within clade 1, the position of U. andersonii is uncertain, occurring as sister to U. laevis + U. stipitata in the MCC tree (Supplementary Data Fig. S1) or sister of the clade in the ML tree (Supplementary Data Fig. S2).
Fig. 10.
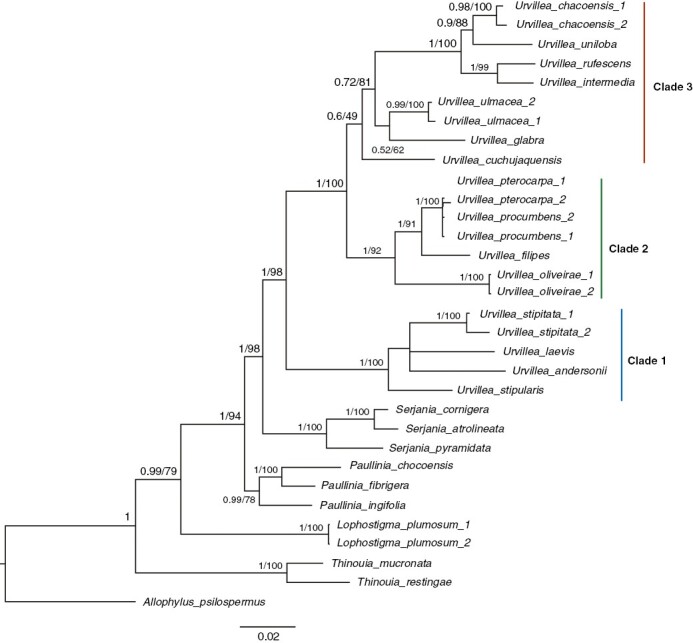
Bayesian 50 % majority-rule consensus tree of Urvillea. Numbers associated with nodes indicate posterior probability/bootstrap support values. The position of U. andersonii within clade 1 (see Supplementary Data Fig. S2).
The ancestor of Urvillea likely had a typical growth (ontogeny 1), and a lobed stem (ontogeny 4) evolved in the ancestor of the lineages leading to clades 1 and 2 + 3 (Fig. 11). Ontogeny 4 is the ancestral condition for clade 1 and clade 3, and a reversal to stems with typical growth occurred in the ancestral of clade 2 (Fig. 11). All other ontogenies evolved once in the genus (Fig. 11). Within clade 1, phloem wedges (ontogeny 2) and fissured stems (ontogeny 3) evolved once. In clade 2, typical growth (ontogeny 1) and ectopic cambia (ontogeny 5) evolved once. Finally, all members of clade 3 retained the ancestral lobed stem ontogeny (Fig. 11).
Fig. 11.
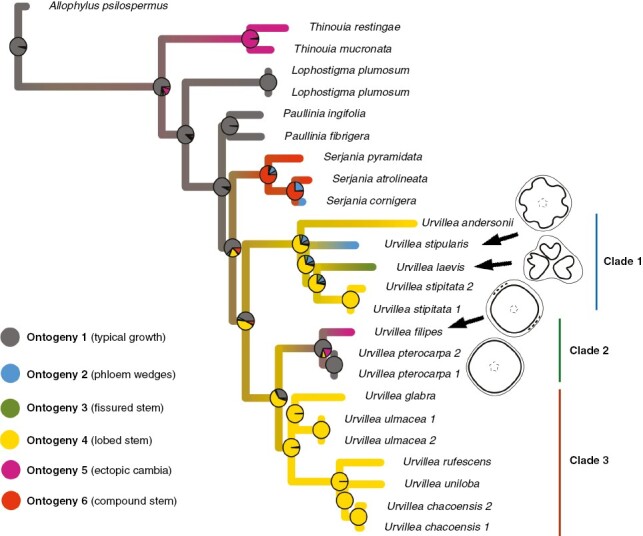
Stochastic character mapping of stem development evolution along the branches of the Urvillea maximum clade credibility tree. The tree was pruned from the full phylogeny (Fig. 10). Phloem wedges, fissured stems and ectopic cambia each evolved once in Urvillea. A reversal to the typical growth occurred in U. pterocarpa. Ontogeny 6 – compound stems include distinct developmental pathways (Cunha Neto, 2023).
DISCUSSION
In this study, the evolution of development of stem anatomy in Urvillea is illustrated for the first time. Four developmental pathways generate vascular variants, and one ontogeny represents typical growth. Below we discuss how these ontogenies develop, how they evolved and their systematic and terminological implications.
Notes on terminology
Radlkofer (1886) classified the vascular variant in Urvillea as ‘corpus lignosum fissum’, which was later translated as ‘fissured vascular cylinder’ (Tamaio et al., 2011). In earlier studies, ‘fissured vascular cylinder’ was generally applied to Urvillea species known to have deeply lobed stems, which could eventually split (Bastos et al., 2016; Pace et al., 2022). However, the classification adopted by Radlkofer (1886) was based mostly on U. laevis, which is now distinctly described with a different ontogeny. Here we adopt the term ‘lobed stem’ for the ancestral ontogeny in Urvillea, characterized by lobed stems that may or may not split (ontogeny 4), and ‘fissured vascular cylinder’ (= fissured stem) was applied only to U. laevis (ontogeny 3), in which vascular tissues become compartmentalized in the stem. This concept of fissured stem and its associated developmental modifications have been described for several families of angiosperms, including Acanthaceae, Malpighiaceae and Passifloraceae (Schenck, 1893; Pfeiffer, 1926; Obaton, 1960; Carlquist, 2001; Acevedo-Rodríguez, 2015 onwards; Cabanillas et al., 2017; Quintanar-Castillo and Pace, 2022). Because this process frequently generates stems with secondary xylem fragmented into different portions, it has also been named ‘dispersed xylem’, ‘xylem dispersed by parenchyma divisions’, ‘wood parts separated by parenchyma proliferation’, ‘fissured xylem’ and ‘fissured stem’ (Metcalfe and Chalk, 1983, p. 198; Carlquist, 1991, 2001; Acevedo-Rodríguez, 2015 onwards; Angyalossy et al., 2015). Because all vascular tissues may become fragmented (including phloem and pith), ‘fissured vascular cylinder’ or ‘fissured stem’ appears more appropriate.
Another terminological issue relates to the presence of ‘ectopic cambia’ (ontogeny 5) in Urvillea. The first evidence of this phenomenon was presented by Ferrucci (2020), who describes the existence of ‘supernumerary cambia’, and their differentiation into ‘vascular cylinders’ in U. filipes. We here adopt ‘ectopic cambia’, as a unifying developmental term that includes ‘successive cambia’, ‘corded’ and ‘neoformations’. These terms have proven useful to identify species for systematic purposes because they are morphologically distinct; however, we here argue for botanical nomenclature that prioritizes developmental processes. Therefore, albeit different morphological configurations at maturity, ‘successive cambia’, ‘corded’ and ‘neoformations’ all represent the appearance of cambia in atypical localities relative to the ancestral mode of secondary growth in seed plants, hence ‘ectopic cambia’.
The distinct ontogeny of U. laevis (ontogeny 3) presents an opportunity to further discuss the utility of the term ‘vascular cylinders’. In Paullinieae, (peripheral) vascular cylinders are usually interpreted as (circular) vascular units each formed from an independent cambium in vascular variants. This is displayed in the following types of vascular variants: divided (Araújo and Costa, 2006; Rizzieri et al., 2021), compound (Van der Walt et al., 1973; Tamaio and Angyalossy, 2009; Chery et al., 2020) and corded stems (Tamaio and Somner, 2010; Borniego and Cabanillas, 2014). Vascular cylinders usually form large, round fragments; therefore, Lopes et al. (2017) used this term to describe the compartmentalized vascular system in U. laevis. Here we demonstrated that the compartmentalized units of U. laevis are the result of disruption of the original vascular cambium and no specific term would be necessary to describe these disrupted vascular tissues. According to Ferrucci (2020), this pattern was observed only in vegetative branches, being absent in reproductive stems. This might be explained by the fact that fissured stems may be macroscopically observed only in later developmental stages. Alternatively, these modifications may be constrained to vegetative branches because they are under greater mechanical stress, therefore triggering such anatomical modifications that may be associated with stem flexibility (Rowe et al., 2004; Isnard and Field, 2015). Additional studies with stems collected in the field, including larger stems from both vegetative and reproductive branches of this plant, would be necessary to expand our understanding of this developmental plasticity.
Phylogenetic inference
This is the first phylogenetic analysis focused on Urvillea. This phylogeny includes novel sequences for 11 Urvillea species (Supplementary Data Table S1) and a combined dataset of 11 genes. This phylogeny is an improvement from previous phylogenies including Urvillea, which utilized few genes or few samples (Acevedo-Rodríguez et al., 2017; Chery et al., 2019; Medeiros et al., 2020). The broader relationships that were recovered in the present phylogeny are highly congruent with previous studies (Acevedo-Rodríguez et al., 2017; Medeiros et al., 2020) including the improved Paullinia phylogeny (with Urvillea species as outgroups) presented by (Chery et al., 2019), which included 11 loci, all genes used in this study. Altogether, these phylogenies corroborate tribe Paullinieae as monophyletic, Thinouia as sister to the rest of Paullinieae, as well as Urvillea as a monophyletic lineage.
The three main clades obtained in this phylogeny largely agree with the section classification of the genus started by Radlkofer (1878) and expanded by Ferrucci (2020). According to Ferrucci (2020), two sections are currently recognized in the genus, section Urvillea (with inflated fruit locules, and three seeds per fruit) and section Stenelytron (with compressed fruit locules). Clade 2 and clade 3 are congruent with section Urvillea (Fig. 10). Clade 1 is congruent with the delimitation of section Stenelytron, although U. stipularis, which is included in section Urvillea based on morphological classification (Ferrucci 2020), was grouped within section Stenelytron in our phylogeny (Table 3). Morphologically, the only features that corroborate the inclusion of U. stipularis in section Stenelytron are related to their stipules (e.g. stipules long, subulate and caducous); all other features corroborate its inclusion in section Urvillea, including inflated fruit, as indicated by Ferrucci (2020). Since the most recent review of the genus by Ferrucci (2020), this species has also been reported outside Espírito Santo state, Brazil for the first time, and now has been reported also in Atlantic Forest in Bahia state. Molecular phylogeny and traditional systematics converge to almost identical section taxonomy in Urvillea.
Table 3.
Summary of infrageneric classification of Urvillea by L. Radlkofer and M. S. Ferrucci and the present study. Species names in bold are included in the present phylogeny.
| Genus | Species | Authority | Radlkofer (1878) | Ferrucci (2020) | This study |
|---|---|---|---|---|---|
| Urvillea | chacoensis | Hunz., | Urvillea | Urvillea | |
| Urvillea | cuchujaquensis | (Ferrucci & Acev.-Rodr.) Acev.-Rodr. & Ferrucci | Urvillea | Urvillea | |
| Urvillea | filipes | Radlk. | Urvillea | Urvillea | |
| Urvillea | glabra | Cambess. | Physelytron | Urvillea | Urvillea |
| Urvillea | intermedia | Radlk. | Physelytron | Urvillea | Urvillea |
| Urvillea | oliveirae | (Ferrucci) Acev.-Rodr. & Ferrucci | Urvillea | Urvillea | |
| Urvillea | paucidentata | Ferrucci | Urvillea | Urvillea | |
| Urvillea | peruviana | Ferrucci | Urvillea | Urvillea | |
| Urvillea | procumbens | (Radlk.) Acev.-Rodr. & Ferrucci |
Urvillea | Urvillea | |
| Urvillea | pterocarpa | (Radlk.) Acev.-Rodr. & Ferrucci | Urvillea | Urvillea | |
| Urvillea | rufescens | Cambess. | Physelytron | Urvillea | Urvillea |
| Urvillea | stipularis | Ferrucci | Urvillea | Stenelytron | |
| Urvillea | triphylla | (Vell.) Radlk. | Physelytron | Urvillea | Urvillea |
| Urvillea | ulmacea | Kunth | Physelytron | Urvillea | Urvillea |
| Urvillea | uniloba | Radlk. | Physelytron | Urvillea | Urvillea |
| Urvillea | andersonii | Ferrucci | Stenelytron | Stenelytron | |
| Urvillea | dasycarpa | Radlk. | Stenelytron | Stenelytron | |
| Urvillea | stipitata | Radlk. | Stenelytron | Stenelytron | Stenelytron |
| Urvillea | laevis | Radlk. | Stenelytron | Stenelytron | Stenelytron |
| Urvillea | venezuelensis | Ferrucci | Stenelytron | Stenelytron |
Evolution of development of stem architectures
Due to the large diversity of vascular variants, stem anatomy has been one important character used to distinguish closely related genera within Paullinieae; however, the evolutionary history of vascular variants has been demonstrated only in the genus Paullinia (Chery et al., 2020). Here we focused on the understudied genus Urvillea. The ancestral stem ontogeny is inferred to be typical growth from which the typical lobed stem evolved. Subsequently, four transitions are inferred in the MCC tree: three ontogenies evolved from lobed stem and one transition occurred from typical growth to ectopic cambia. This evolutionary scenario demonstrates the large developmental plasticity of the cambium in such a small genus (20 species) and corroborates a scenario of repeated evolution of complex anatomies within Paullinieae lianas, as already demonstrated for Paullinia (Chery et al., 2020). Given that species with a lobed stem (ancestral ontogeny) start stem development with lobed stems, both Paullinia and Urvillea present in their evolutionary history shifts from lobed structure to circular/typical growth (ontogeny 1 in Urvillea), which is interpreted as reversals to the ancestral state in the node subtending the diversification of Paullinieae.
Diverse developmental modifications generate distinct vascular variants
Typical growth in Urvillea is observed solely in the two rhizomatous plants with herbaceous prostrate shoots (U. pterocarpa and U. procumbens). This reversal to typical growth is likely explained by paedomorphosis, as all ontogenies undergo some period of typical development, which then characterizes the final developmental stage for these two species. On the other hand, all other Urvillea species are characterized as herbaceous or woody vines (Ferrucci, 2020) and have one or another type of vascular variant.
As a rule, developmental modifications during secondary growth (and not primary growth) promote the evolution of development in stems of Urvillea. The four vascular variants result from developmental modifications characterizing anatomical patterns from two categories (Cunha Neto, 2023): (1) cambial variants, through differential cambial activity producing unequal rates and spatial distribution of vascular tissue governed by a single cambium; and (2) ectopic cambia, that produce multiple cambia nested in the stem. Here, cambial variants include the formation of lobed stems, phloem wedges and fissured stems. In Urvillea, phloem wedges are an intermediate stage in the development of fissured stems (ontogeny 3) which corroborates the findings of Quintanar-Castillo and Pace (2022), indicating that phloem wedges have facilitated the evolution of more complex vascular variants such as fissured stems and interxylary phloem. However, the development of fissured stems in U. laevis is not entirely similar to that described in Malpighiaceae (Cabanillas et al., 2017; Quintanar-Castillo and Pace, 2022) since Malpighiaceae lianas may have the vascular system dissected not only due to phloem wedges and through proliferation of parenchyma cells (= disruptive parenchyma) as observed in U. laevis, but also through the inclusion of phloem wedges constituting a type of interxylary phloem (Quintanar-Castillo and Pace, 2022) or through the formation of new cambia from this proliferated parenchyma (= interxylary cambia; Pace et al., 2018). If compared with U. laevis, the dissection of vascular tissue through disruptive parenchyma is more conspicuous in Malpighiaceae (Cabanillas et al., 2017).
In the case of lobed stems (ontogeny 4), differential cambial activity produces vascular tissues at uneven rates, which generates lobes and furrows. In Urvillea leaves are alternate, and lobes form at the node and divide and fuse above and below it, similar to species of Paullinia. This is also the case in some Malvaceae (Luna-Márquez et al., 2021), while in other species the lobes arise in the furrows (Gama and Oskolski, 2021). Because this differential cambial activity is more pronounced and continues for a longer period, stem split may also occur in some Urvillea. In this scenario, lobed structure is observed as an intermediate stage prior to stem splitting, as also suggested by Schenck (1893). Despite many taxa developing a lobed stem, only some of them actually split. This phenomenon has been associated with distinct developmental processes (Fisher and Ewers, 1992; Caballé, 1993, 1994; Alyonkin and Barykina, 2020), including larger and wider rays in the furrows that accommodate the growth differences among cambium segments and lobes, as also observed in some Urvillea. Stem splitting is a phenomenon observed also in other aerial and subterraneous organs of plants with distinct habits (e.g. hemicryptophytes) (Jost, 1890; Fritz and Saukel, 2011), and has been suggested as an adaptation to hydraulic compartmentalization, such as in plants growing in harsh environments and unstable ground (Schenk, 1999; Lambert et al., 2011; Alyonkin and Barykina, 2020). Such longitudinal splitting in stems of woody vines has been hypothesized as a way of vegetative reproduction (cloning), which can be an advantage in unstable forest conditions (Caballé, 1994). On the other hand, some authors suggest that splitting could be a mere consequence of ageing or a sign of senescence, without any adaptive advantage (Schenk, 1999). In Urvillea, stem splitting is also a late ontogenetic phenomenon, which may indicate that this trait may persist in a lineage as long as it is not entirely detrimental to the species.
Position of lobed structure and phloem wedges in relation to leaf orthostichy in U. uniloba
The position of phloem wedges and its association with other structures in the shoot has been demonstrated in different families, including Sapindaceae. In general, the position of phloem wedges is not random, occurring either below or alternating with nodes (Pace et al., 2011; Cabanillas et al., 2017; Quintanar-Castillo and Pace, 2022), but they have also been indicated to arise randomly in the stem circumference (e.g. Serjania, Sapindaceae; Bastos et al., 2016) or coinciding with the region delimited by the interfascicular cambium (e.g. Paullinia, Sapindaceae; Chery et al., 2020). In U. uniloba, stems may present pronounced lobes and furrows, which were observed under the leaf and/or branch nodes, while phloem wedges not as pronounced can be found in the lobes, i.e. between leaf insertions. The distribution of phloem wedges between leaf insertions is also observed in Malpighiaceae (Quintanar-Castillo and Pace, 2022) and Bignoniaceae (Pace et al., 2011), although they may also occur under the nodes in other lineages of Malpighiaceae (Cabanillas et al., 2017). The correlation between the formation of phloem wedges and leaf position has been hypothesized as an indication of their association with auxin regulation (Angyalossy et al., 2015; Quintanar-Castillo and Pace, 2022). In Paullinieae lianas, the formation of lobes, such as in U. uniloba, usually coincides with the accelerated activity of the fascicular cambium, whereas the reduced cambium activity in the interfascicular cambium generates the furrows. The interplay between auxin flux and other cambium-derived signals is also likely responsible for the differential activity of fascicular- and interfascicular cambium (Tomescu and Groover, 2019), another evidence of hormonal activity underlying vascular development and possibly complex morphologies, as in the case of phloem wedges.
Diversity of vascular variants in Urvillea and its systematic implications for Paullinieae
Until now, only two types of vascular variants had been described from Urvillea (i.e. lobed stem and fissured stem; Pace et al., 2022). Although it is a surprising result that Urvillea with only 20 species present four vascular variants, these ontogenies have also been reported for other genera in Paullinieae, except ontogeny 3 with the formation of fissured stem. Within Paullinieae lianas, this developmental pathway may be similar to the ontogeny observed in Serjania piscatoria (Schenck 1893; I. L. Cunha Neto and N. F. Marques, pers. observ.). The pronounced and regularly distributed phloem wedges with limiting rays (ontogeny 2) is also present in some species of Serjania (Rajput et al., 2021) and Paullinia (P. Acevedo-Rodríguez, pers. observ.), while lobed stems (ontogeny 4) and ectopic cambia (ontogeny 5) are shared with both Paullinia and Serjania (Bastos et al., 2016; Cunha Neto et al., 2018; Pace et al., 2022) and with Thinouia (Tamaio and Somner, 2010) if considering the de novo formation of peripheral vascular cylinders as a type of ectopic cambia (Cunha Neto, 2023). Therefore, the only ontogeny exclusive to Urvillea is the true fissured stem, observed exclusively in the stem of U. laevis. Following this classification, Urvillea now stands out as the third most diverse genus in number of vascular variants within Paullinieae, following the genus Serjania (~300 species) (which contains virtually all types) and Paullinia (~200 species), the second most diverse lineage with most types except the divided vascular cylinder and compound stems with a central vascular cylinder and 8–12 peripheral vascular cylinders (Acevedo-Rodríguez, 2015 onwards; Pace et al., 2022).
The description of ectopic cambia in U. filipes is remarkable as this is the third genus to be described with this ontogeny in the family, following the description for Serjania pernambucensis and some species of Paullinia (Cunha Neto et al., 2018). In terms of origin and development, ectopic cambia in U. filipes are more similar to Paullinia species (Cunha Neto et al., 2018), as ectopic cambia originate from a meristematic zone at the boundary between the fibrous pericycle and the endodermis; Serjania pernambucensis produces new cambia towards the phloem (Cunha Neto et al., 2018), as are the new cambia formed in ‘corded stems’ (Tamaio and Somner, 2010; Borniego and Cabanillas, 2014). In addition, ectopic cambia in U. filipes form discrete vascular increments, and may even produce two meristematic zones with a new cambium in both the inner and the outer boundary of the fibrous pericycle, as also demonstrated for Paullinia (Cunha Neto et al., 2018). It is important to note that Ferrucci (2020) had already described the existence of ‘supernumerary cambia’ (= ‘cámbiumes supernumerarios’ in Spanish) in U. filipes, which corresponds to the presence of ectopic cambia in our study.
CONCLUSIONS
Here we present the first molecular phylogeny of Urvillea, which is confirmed monophyletic as proposed by Acevedo et al. (2017), and closer to Serjania than to Paullinia as was proposed by Chery et al. (2019). This new phylogeny is used to explore the morphological evolution of stem patterns. Stem ontogeny in Urvillea is surprisingly diverse. Five developmental pathways including four vascular variants and typical growth (ontogeny 1) account for stem vascular diversity in the genus. Phloem wedges (ontogeny 2), one of the vascular variants, are observed as either the final or intermediate stages in the formation of more complex vascular variants. Phloem wedges with disrupted cambium continuity generate fissured stems (ontogeny 3), which is dissociated here from lobed stems (ontogeny 4, ancestral ontogeny), whose lobes may split longitudinally. Given the description of ectopic cambia (ontogeny 5) in Urvillea, our study highlights this developmental process as one of the major phenomena generating vascular patterns in Paullinieae lianas, as it is observed also in the other four genera with vascular variants in the family. Most stem developments in Urvillea are shared with Paullinia and Serjania. These findings confirm the convergent evolution of vascular variants in Paullinieae; however, it is still unknown whether these independent instances are driven by similar and distinct genetic mechanisms.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Table S1: list of genes, pairwise identity, alignment length, models for Bayesian analyses and accession number for all specimens used for phylogenetic studies. Table S2: model comparisons for character evolution. Dataset S1: alignment used for phylogenetic analysis. Figure S1: Bayesian maximum clade credibility tree of Urvillea. Values associated with nodes indicate posterior probability. Figure S2: maximum likelihood tree of Urvillea. Values associated with nodes indicate the likelihood bootstrap value.
ACKNOWLEDGEMENTS
The first author dedicates this article to Dr Neusa Tamaio (Rio de Janeiro Botanical Garden) for her mentorship and inspiration to study Sapindaceae lianas. We acknowledge Dr Tamaio’s contribution by sharing samples, slides and images of Urvillea glabra, U. stipitata and U. rufescens. We thank the curators of various herbaria (ALCB, HURB, RBR, US) who allowed collections of specimens for anatomical studies. The authors declare that there are no conflicts of interest.
Contributor Information
Israel L Cunha Neto, School of Integrative Plant Sciences and L. H. Bailey Hortorium, Cornell University, Ithaca, NY 14853, USA.
Yanã C Rizzieri, School of Integrative Plant Sciences and L. H. Bailey Hortorium, Cornell University, Ithaca, NY 14853, USA.
Pablo A Cabanillas, Cátedra de Dendrología, Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, Diagonal 113 No. 469 esquina 117, CP 1900, La Plata, Argentina.
Fabiano M Martins, Universidade Federal do Recôncavo da Bahia, Centro de Ciências Agrárias, Ambientais e Biológicas, CP 44380-000, Cruz das Almas, BA, Brazil.
Natália F Marques, Museu Nacional, Universidade Federal do Rio de Janeiro (UFRJ), Quinta da Boa Vista, São Cristóvão, 20940-040, s.n. Rio de Janeiro, RJ, Brazil.
Genise V Somner, Universidade Federal Rural do Rio de Janeiro, Instituto de Ciências Biológicas e da Saúde, Departamento de Botânica, CP 74582, 23851-970 Rio de Janeiro, RJ, Brazil.
Pedro Acevedo-Rodríguez, Department of Botany, Smithsonian National Museum of Natural History, 10th Street and Constitution Avenue NW, Washington, DC 20560, USA.
Joyce G Onyenedum, School of Integrative Plant Sciences and L. H. Bailey Hortorium, Cornell University, Ithaca, NY 14853, USA.
FUNDING
This work was supported by startup laboratory funds from Cornell University (J.G.O.).
LITERATURE CITED
- Acevedo-Rodríguez P. 1993. Systematics of Serjania (Sapindaceae) Part I: A revision of Serjania sect. Platycoccus. Memoirs of the New York Botanical Garden 1: 2–91. [Google Scholar]
- Acevedo-Rodríguez P. 2015 onwards. Lianas and climbing plants of the Neotropics. https://naturalhistory.si.edu/research/botany/research/lianas-and-climbing-plants-neotropics(26 July 2023, date last accessed).
- Acevedo-Rodríguez P, Wurdack KJ, Ferrucci MS, et al. 2017. Generic relationships and classification of tribe Paullinieae (Sapindaceae) with a new concept of supertribe Paulliniodae. Systematic Botany 42: 96–114. doi: 10.1600/036364417x694926. [DOI] [Google Scholar]
- Alyonkin VY, Barykina RP.. 2020. Morphological and anatomical characteristics of Mertensia maritima (L.) S.F. Gray supralittoral of the White Sea and Russian Far East coast. Wulfenia 27: 289–302. [Google Scholar]
- Angyalossy V, Angeles G, Pace MR, et al. 2012. An overview of the anatomy, development and evolution of the vascular system of lianas. Plant Ecology and Diversity 5: 167–182. doi: 10.1080/17550874.2011.615574. [DOI] [Google Scholar]
- Angyalossy V, Angeles G, Pace M, Lima AC.. 2015. Liana anatomy: a broad perspective on structural evolution of the vascular system. In: Schnitzer SA, Bongers F, Burnham RJ, eds. Ecology of lianas. Chichester: John Wiley, 253–287. [Google Scholar]
- Araújo GUC, Costa CG.. 2006. Cambial variant in the stem of Serjania corrugata (Sapindaceae). IAWA Journal 27: 269–280. [Google Scholar]
- Barbosa ACF, Pace MR, Witovisk L, Angyalossy V.. 2010. A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA Journal 31: 373–383. doi: 10.1163/22941932-90000030. [DOI] [Google Scholar]
- Barbosa ACF, Gerolamo CS, Lima AC, Angyalossy V, Pace MR.. 2021. Polishing entire stems and roots using sandpaper under water: an alternative method for macroscopic analyses. Applications in Plant Sciences 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos CL, Tamaio N, Angyalossy V.. 2016. Unravelling roots of lianas: a case study in Sapindaceae. Annals of Botany 118: 733–746. doi: 10.1093/aob/mcw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borniego ML, Cabanillas PA.. 2014. Desarrollo de la variante cambial en Serjania meridionalis (Sapindaceae, Paullinieae). Darwiniana 2: 144–153. [Google Scholar]
- Caballé G. 1993. Liana structure, function and selection: a comparative study of xylem cylinder in Africa and America. Botanical Journal of the Linnean Society 113: 41–60. doi: 10.1111/j.1095-8339.1993.tb00328.x. [DOI] [Google Scholar]
- Caballé G. 1994. Ramet proliferation by longitudinal splitting in the Gabonese rain forest liana Dalhousiea africana S. Moore (Papilionaceae). Biotropica 26: 266–275. doi: 10.2307/2388847. [DOI] [Google Scholar]
- Cabanillas PA, Pace MR, Angyalossy V.. 2017. Structure and ontogeny of the fissured stems of Callaeum (Malpighiaceae). IAWA Journal 38: 49–66. doi: 10.1163/22941932-20170156. [DOI] [Google Scholar]
- Carlquist S. 1991. Anatomy of vine and liana stems: a review and synthesis. In: Putz FE., Mooney HA, eds. The biology of vines. Cambridge: Cambridge University Press, 53–72. [Google Scholar]
- Carlquist S. 2001. Comparative wood anatomy. Systematic, ecological and evolutionary aspects of dicotyledon wood. Berlin: Springer. [Google Scholar]
- Carlquist S. 2013. More woodiness/less woodiness: evolutionary avenues, ontogenetic mechanisms. International Journal of Plant Sciences 174: 964–991. doi: 10.1086/670400. [DOI] [Google Scholar]
- Chery JG, Sass C, Specht CD.. 2017. Development of single-copy nuclear intron markers for species-level phylogenetics: case study with Paullinieae (Sapindaceae). Applications in Plant Sciences 5: 1700051. doi: 10.3732/apps.1700051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chery JG, Acevedo-Rodríguez P, Rothfels CJ, Specht CD.. 2019. Phylogeny of Paullinia L. (Paullinieae Sapindaceae), a diverse genus of lianas with dynamic fruit evolution. Molecular Phylogenetics and Evolution 140: 106577. doi: 10.1016/j.ympev.2019.106577. [DOI] [PubMed] [Google Scholar]
- Chery JG, Pace MR, Acevedo-Rodríguez P, Specht CD, Rothfels CJ.. 2020. Modifications during early plant development promote the evolution of nature’s most complex woods. Current Biology 30: 237–244.e2. doi: 10.1016/j.cub.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Cunha Neto IL. 2023. Vascular variants in seed plants – a developmental perspective. AoB PLANTS 15: 1–15. doi: 10.1093/aobpla/plad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha Neto IL, Martins FM, Somner GV, Tamaio N.. 2018. Successive cambia in liana stems of Paullinieae and their evolutionary significance in Sapindaceae. Botanical Journal of the Linnean Society 186: 66–88. doi: 10.1093/botlinnean/box080. [DOI] [Google Scholar]
- Cunha Neto IL, Pace MR, Hernández-Gutiérrez R, Angyalossy V.. 2022. Linking the evolution of development of stem vascular system in Nyctaginaceae and its correlation to habit and species diversification. EvoDevo 13: 1–24. doi: 10.1186/s13227-021-00190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha Neto IL, Hall BT, Lanba AR, Blosenski JD, Onyenedum JG.. 2023. Laser ablation tomography (LATscan) as a new tool for anatomical studies of woody plants. New Phytologist 239: 429–444. doi: 10.1111/nph.18831. [DOI] [PubMed] [Google Scholar]
- Demesure B, Sodzi N, Petit RJ.. 1995. A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Molecular Ecology 4: 129–131. doi: 10.1111/j.1365-294x.1995.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL.. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Fazekas AJ, Burgess KS, Kesanakurti PR, et al. 2008. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS One 3: e2802. doi: 10.1371/journal.pone.0002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci MS. 2020. Taxonomic revision of Urvillea (Sapindaceae, Paullinieae): an American genus. Boletin de la Sociedad Argentina de Botanica 55: 53–130. [Google Scholar]
- Fisher JB, Ewers FW.. 1991. Structural responses to stem injury in vines. In: Putz FE, Mooney HA, eds. The biology of vines. Cambridge: Cambridge University Press, 99–124. [Google Scholar]
- Fisher JB, Ewers FW.. 1992. Xylem pathways in liana stems with variant secondary growth. Botanical Journal of the Linnean Society 108: 181–202. doi: 10.1111/j.1095-8339.1992.tb01640.x. [DOI] [Google Scholar]
- Fritz E, Saukel J.. 2011. Interxylary cork of Saussurea discolor and S. pygmaea (Asteraceae). Biologia 66: 454–457. doi: 10.2478/s11756-011-0030-5. [DOI] [Google Scholar]
- Gama G, Oskolski A.. 2021. Stem anatomy of Grewia caffra (Malvaceae): an uncommon cambial variant in the order Malvales. Plant Systematics and Evolution 307: 21. [Google Scholar]
- Hoffman LA, Tomescu AMF.. 2013. An early origin of secondary growth: Franhueberia gerriennei gen. et sp. nov. from the Lower Devonian of Gaspé (Quebec, Canada). American Journal of Botany 100: 754–763. doi: 10.3732/ajb.1300024. [DOI] [PubMed] [Google Scholar]
- Isnard S, Feild TS.. 2015. The evolution of angiosperm lianescence: a perspective from xylem structure-function In: Schnitzer SA, Bongers F, Burnham RJ, eds. Ecology of lianas. Chichester: John Wiley, 221–250. [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York: McGraw-Hill. [Google Scholar]
- Jost L. 1890. Die Zerkluftungen einiger Rhizome und Wurzeln. Botanische Zeitung 48: 433–446. [Google Scholar]
- Kraus JE, Arduin M.. 1997. Manual básico de métodos em morfologia vegetal. Rio de Janeiro: Editora Universidade Rural. [Google Scholar]
- Lambert SE, Jones CS, Schenk HJ.. 2011. Distribution of axis-splitting in Mojave Desert shrub species along an elevational gradient. Journal of Arid Environments 75: 106–111. doi: 10.1016/j.jaridenv.2010.09.019. [DOI] [Google Scholar]
- Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30: 3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal MOL, Nascimento LB, Coutinho AJ, Tamaio N, Brandes AFN.. 2020. Development of external vascular cylinders (neoformations) in stems and roots of Chiococca alba (L.) Hitchc. (Rubiaceae). Flora 264: 151569. [Google Scholar]
- Leme CLD, Cunha Neto IL, Angyalossy V.. 2020. How the neotropical liana Machaerium multifoliolatum (Fabaceae) develop their distinctive flattened stems? Flora 269: 151629. [Google Scholar]
- Lopes WAL, De Souza LA, De Almeida OJG.. 2017. Procambial and cambial variants in Serjania and Urvillea species (Sapindaceae: Paullinieae). Journal of the Botanical Research Institute of Texas 11: 421–432. [Google Scholar]
- Luna-Márquez L, Sharber WV, Whitlock BA, Pace MR.. 2021. Ontogeny, anatomical structure and function of lobed stems in the evolution of the climbing growth form in Malvaceae (Byttneria Loefl.). Annals of Botany 128: 859–874. doi: 10.1093/aob/mcab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros H, de Carvalho Lopes J, Acevedo-Rodríguez P, Forzza RC.. 2020. A new species of Thinouia (Paullinieae, Sapindaceae) from the Amazon and its phylogenetic placement. PhytoKeys 165: 115–126. doi: 10.3897/phytokeys.165.57341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe CR, Chalk L.. 1950. Anatomy of the dicotyledons. Oxford: Clarendon Press. [Google Scholar]
- Metcalfe CR, Chalk L.. 1983. Anatomy of the dicotyledons. Vol. 2. Wood structure and conclusion of the general introduction. Oxford: Clarendon Press. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T.. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE): 1–8.
- Nejapa R, Cabanillas PA, Pace MR.. 2021. Cortical origin of the successive cambia in the stems of the charismatic temperate lianescent genus Wisteria (Fabaceae) and its systematic importance. Botanical Journal of the Linnean Society 199: 667–677. doi: 10.1093/botlinnean/boab091. [DOI] [Google Scholar]
- O’Brien TP, Feder N, McCully MW.. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 368–373. [Google Scholar]
- Obaton M. 1960. Les lianes ligneuses à structure anormale des forêts denses d’Afrique Occidentale. Annales des Sciences Naturelles Botanique et Biologie Végétale 12: 1–20. [Google Scholar]
- Onyenedum JG, Pace MR.. 2021. The role of ontogeny in wood diversity and evolution. American Journal of Botany 108: 2331–2355. doi: 10.1002/ajb2.1801. [DOI] [PubMed] [Google Scholar]
- Pace MR, Lohmann LG, Angyalossy V.. 2009. The rise and evolution of the cambial variant in Bignonieae (Bignoniaceae). Evolution and Development 11: 465–479. doi: 10.1111/j.1525-142X.2009.00355.x. [DOI] [PubMed] [Google Scholar]
- Pace MR, Lohmann LG, Angyalossy V.. 2011. Evolution of disparity between the regular and variant phloem in Bignonieae (Bignoniaceae). American Journal of Botany 98: 602–618. doi: 10.3732/ajb.1000269. [DOI] [PubMed] [Google Scholar]
- Pace MR, Acevedo-Rodríguez P, Amorim AM, Angyalossy V.. 2018. Ontogeny, structure and occurrence of interxylary cambia in Malpighiaceae. Flora 241: 46–60. doi: 10.1016/j.flora.2018.02.004. [DOI] [Google Scholar]
- Pace MR, Gerolamo CS, Onyenedum JG, et al. 2022. The wood anatomy of Sapindales: diversity and evolution of wood characters. Revista Brasileira de Botanica 45: 283–340. [Google Scholar]
- Pellissari LCO, Barros CF, Medeiros H, Tamaio N.. 2018. Cambial patterns of Paullinia (Sapindaceae) in southwestern Amazonia, Brazil. Flora 246–247: 71–82. doi: 10.1016/j.flora.2018.07.002. [DOI] [Google Scholar]
- Pennell MW, Eastman JM, Slater GJ, et al. 2014. geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 30: 2216–2218. doi: 10.1093/bioinformatics/btu181. [DOI] [PubMed] [Google Scholar]
- Pfeiffer H. 1926. Das Abnorme Dickenwachstum – Handbuch der Pflanzenanatomie. Band IX. Berlin: Gebrüder Borntraeger. [Google Scholar]
- Quintanar-Castillo A, Pace MR.. 2022. Phloem wedges in Malpighiaceae: origin, structure, diversification, and systematic relevance. EvoDevo 13: 11. doi: 10.1186/s13227-022-00196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2022. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Radlkofer L. 1878. Ueber Sapindus und damit in Zusammenhang stehende Pflanzen. In: Sitzungsberichte der Mathematisch-Physikalisch Classe der. k. b. Akademie der Wissenschaften zu München, Vol. 8, 221–408. [Google Scholar]
- Radlkofer L. 1886. Ergänzungen zur Monographie der Sapindaceen-Gattung Serjania. München: K. B. Akademie. [Google Scholar]
- Radlkofer L. 1931. Sapindaceae. In: Engler A, ed. Das Pflanzenreich IV, 165, Heft 98a–h. Leipzig: W. Engelmann, 1– 1539. [Google Scholar]
- Ragni L, Greb T.. 2018. Secondary growth as a determinant of plant shape and form. Seminars in Cell and Developmental Biology 79: 58–67. doi: 10.1016/j.semcdb.2017.08.050. [DOI] [PubMed] [Google Scholar]
- Rajput KS, Gondaliya AD, Moya R.. 2021. Structure of the secondary xylem and development of a cambial variant in Serjania mexicana (Sapindaceae). IAWA Journal 43: 103–115. doi: 10.1163/22941932-bja10075. [DOI] [Google Scholar]
- Rambaut A, Suchard MA, Xie D, Drummond AJ.. 2014. Tracer v1.6. http://beast.bio.ed.ac.uk/Tracer [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. doi: 10.1111/j.2041-210x.2011.00169.x. [DOI] [Google Scholar]
- Revell LJ. 2013. Two new graphical methods for mapping trait evolution on phylogenies. Methods in Ecology and Evolution 4: 754–759. doi: 10.1111/2041-210x.12066. [DOI] [Google Scholar]
- Rizzieri YC, Brandes AFN, Cunha Neto IL, Somner GV, et al. . 2021. Ontogeny of divided vascular cylinders in Serjania: the rise of a novel vascular architecture in Sapindaceae. IAWA Journal 42(2): 121–133. [Google Scholar]
- Rowe N, Isnard S, Speck T.. 2004. Diversity of mechanical architectures in climbing plants: an evolutionary perspective. Journal of Plant Growth Regulation 23: 108–128. doi: 10.1007/s00344-004-0044-0. [DOI] [Google Scholar]
- Schenck H. 1893. Beiträge zur Biologie und Anatomie der Lianen im Besonderen der in Brasilien einheimische Arten. In: Schimper AFW, Fischer G, eds. Botanische Mittheilungen aus der Tropens. Jena: Gustav Fischer. [Google Scholar]
- Schenk HJ. 1999. Clonal splitting in desert shrubs. Plant Ecology 141: 41–52. [Google Scholar]
- Spicer R, Groover A.. 2010. Evolution of development of vascular cambia and secondary growth. New Phytologist 186: 577–592. doi: 10.1111/j.1469-8137.2010.03236.x. [DOI] [PubMed] [Google Scholar]
- Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution 4: vey016. doi: 10.1093/ve/vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J.. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- Tamaio N, Angyalossy V.. 2009. Variação cambial em Serjania caracasana (Sapindaceae): enfoque na adequação terminológica. Rodriguésia 60: 651–666. doi: 10.1590/2175-7860200960311. [DOI] [Google Scholar]
- Tamaio N, Somner GV.. 2010. Development of corded vascular cylinder in Thinouia restingae Ferruci & Somner (Sapindaceae: Paullinieae). Journal of the Torrey Botanical Society 137: 319–326. doi: 10.3159/10-ra-047.1. [DOI] [Google Scholar]
- Tamaio N, Neves MF, Brandes AFN, Vieira RC.. 2011. Quantitative analyses establish the central vascular cylinder as the standard for wood-anatomy studies in lianas having compound stems (Paullinieae: Sapindaceae). Flora 206: 987–996. [Google Scholar]
- Tomescu AMF, Groover AT.. 2019. Mosaic modularity: an updated perspective and research agenda for the evolution of vascular cambial growth. New Phytologist 222: 1719–1735. doi: 10.1111/nph.15640. [DOI] [PubMed] [Google Scholar]
- Van der Walt JJA, Van der Schijff HP, Schweickerdt HG.. 1973. Anomalous secondary growth in the stem of lianas Mikania cordata (Burm. F.) Robins (Compositae) and Paullinia pinnata Linn. (Sapindaceae). Kirkia 9: 109–138. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J.. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Shinsky JJ, White TJ, eds. PCR protocols: a guide to methods and applications. New York: Academic Press, 315–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


