Abstract
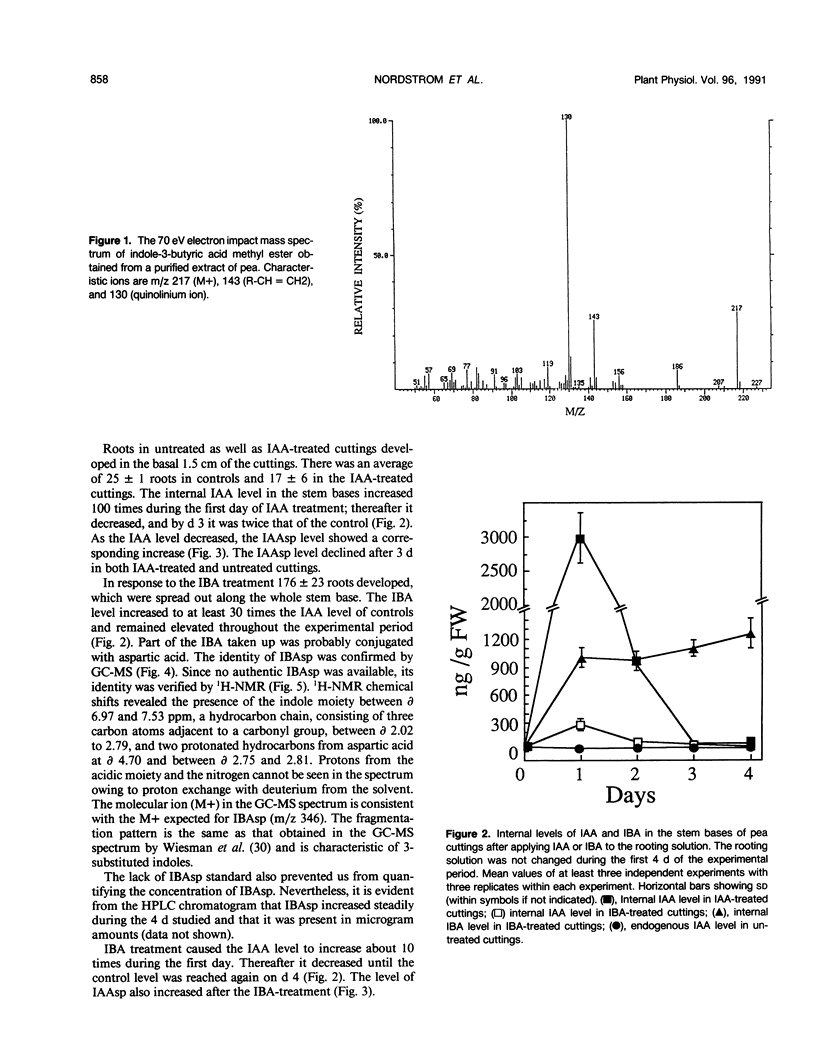
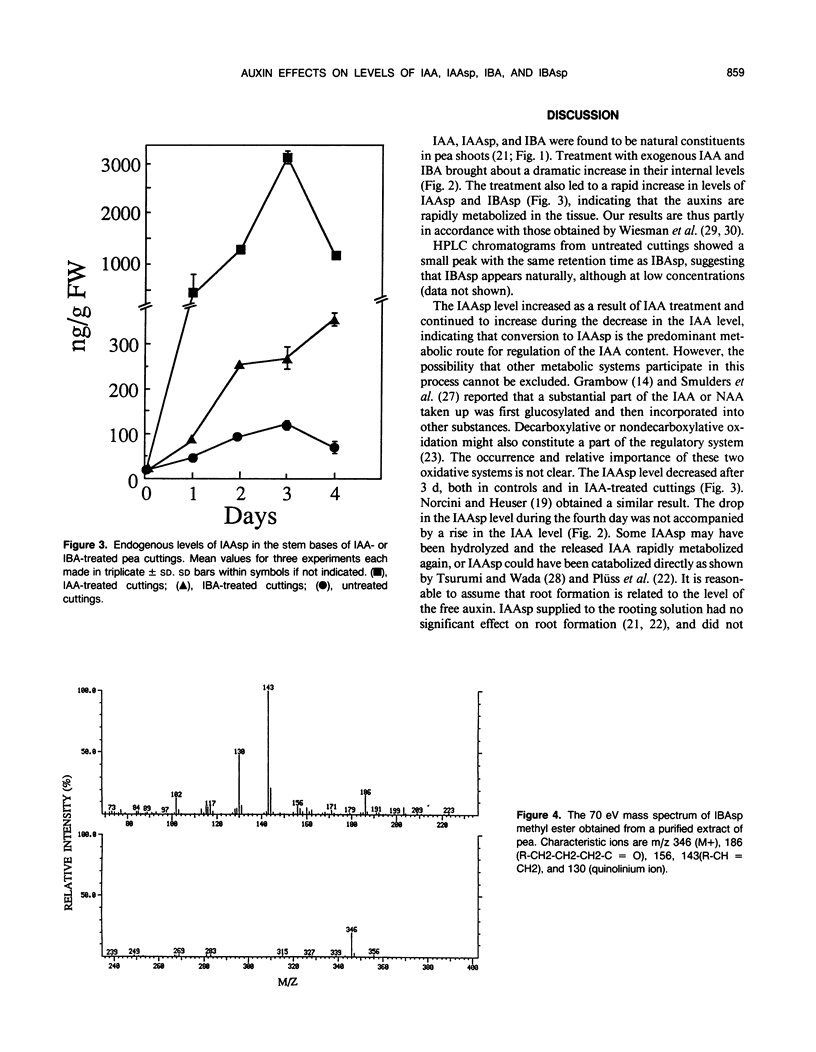
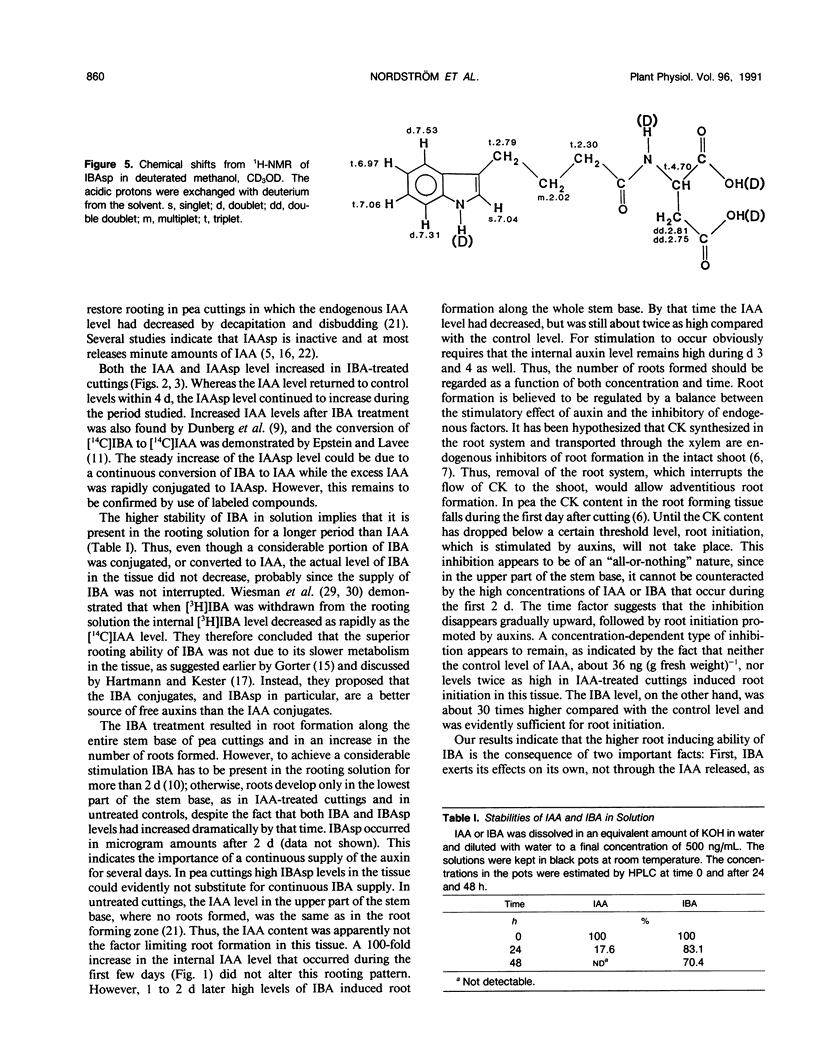
The influence of exogenous indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) on the internal levels of these auxins was studied during the first 4 days of adventitious root formation in cuttings of Pisum sativum L. The quantitations were done by high performance liquid chromatography with spectrofluorometric detection. IBA, identified by combined gas chromatography-mass spectrometry (GC-MS), was found to naturally occur in this plant material. The root inducing ability of exogenous IBA was superior to that of IAA. The IAA level in the tissue increased considerably on the first day after application of IAA, but rapidly decreased again, returning to a level twice the control by day 3. The predominant metabolic route was conjugation with aspartic acid, as reflected by the increase in the level of indole-3-acetylaspartic acid. The IBA treatment resulted in increases in the levels of IBA, IAA, and indole-3-acetylaspartic acid. The IAA content rapidly returned to control levels, whereas the IBA level remained high throughout the experimental period. High amounts of indole-3-butyrylaspartic acid were found in the tissue after feeding with IBA. The identity of the conjugate was confirmed by 1H-nuclear magnetic resonance and GC-MS. IBA was much more stable in solution than IAA. No IAA was detected after 48 hours, whereas 70% IBA was still recovered after this time. The relatively higher root inducing ability of IBA is ascribed to the fact that its level remained elevated longer than that of IAA, even though IBA was metabolized in the tissue. Adventitious root formation is discussed on the basis of these findings.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae W. A., Good N. E. Studies on 3-Indoleacetic Acid Metabolism. IV. Conjugation with Aspartic Acid and Ammonia as Processes in the Metabolism of Carboxylic Acids. Plant Physiol. 1957 Nov;32(6):566–572. doi: 10.1104/pp.32.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae W. A., Good N. E. The Formation of Indoleacetylaspartic Acid in Pea Seedlings. Plant Physiol. 1955 Jul;30(4):380–382. doi: 10.1104/pp.30.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H. Gas chromatographic analysis of acidic indole auxins in Nicotiana. Plant Physiol. 1969 Feb;44(2):267–271. doi: 10.1104/pp.44.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Meudt W. J., Cohen J. D. Indole-3-acetic Acid (IAA) and IAA Conjugates Applied to Bean Stem Sections: IAA Content and the Growth Response. Plant Physiol. 1983 Sep;73(1):130–134. doi: 10.1104/pp.73.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R. P., Peterson M. D., Good N. E. Biological activities of indoleacetylamino acids and their use as auxins in tissue culture. Plant Physiol. 1980 May;65(5):761–767. doi: 10.1104/pp.65.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. M., Hamilton R. H. A rapid isotope dilution method for analysis of indole-3-acetic acid and indoleacetyl aspartic acid from small amounts of plant tissue. Biochem Biophys Res Commun. 1982 Jun 15;106(3):1035–1041. doi: 10.1016/0006-291x(82)91815-0. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcini J. G., Heuser C. W. Changes in the Level of [C]Indole-3-Acetic Acid and [C]Indoleacetylaspartic Acid during Root Formation in Mung Bean Cuttings. Plant Physiol. 1988 Apr;86(4):1236–1239. doi: 10.1104/pp.86.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman Z., Riov J., Epstein E. Characterization and Rooting Ability of Indole-3-Butyric Acid Conjugates Formed during Rooting of Mung Bean Cuttings. Plant Physiol. 1989 Nov;91(3):1080–1084. doi: 10.1104/pp.91.3.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]


