Abstract
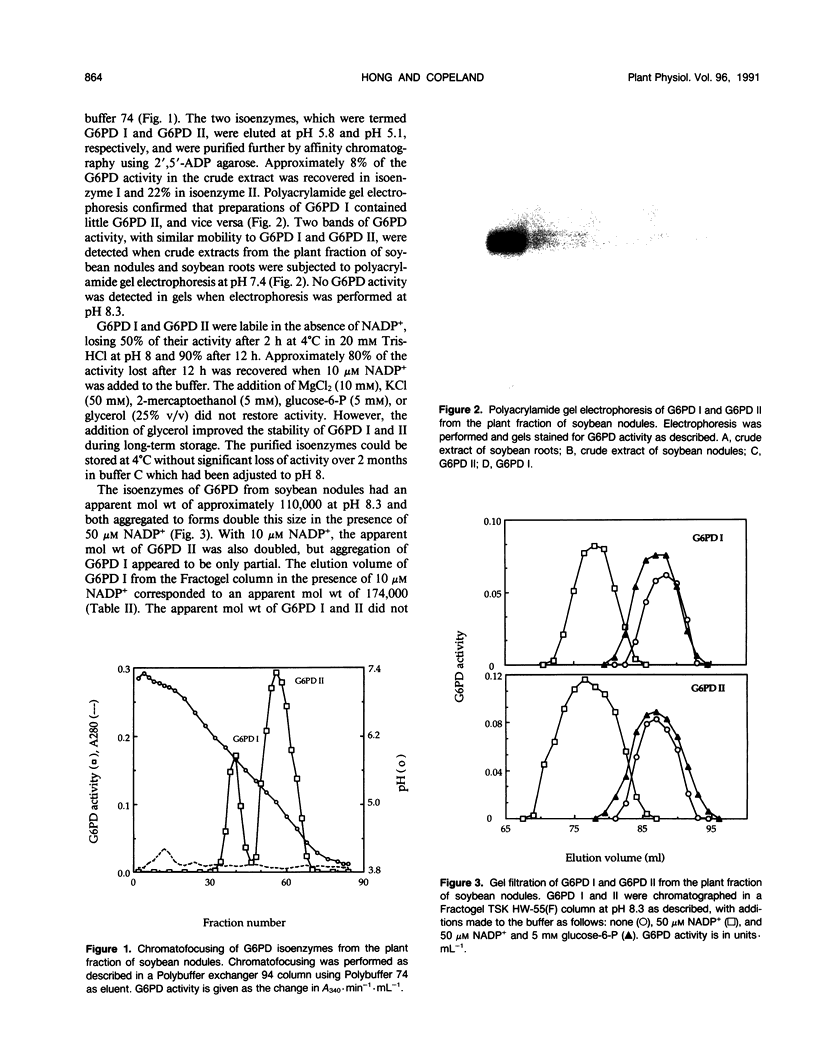
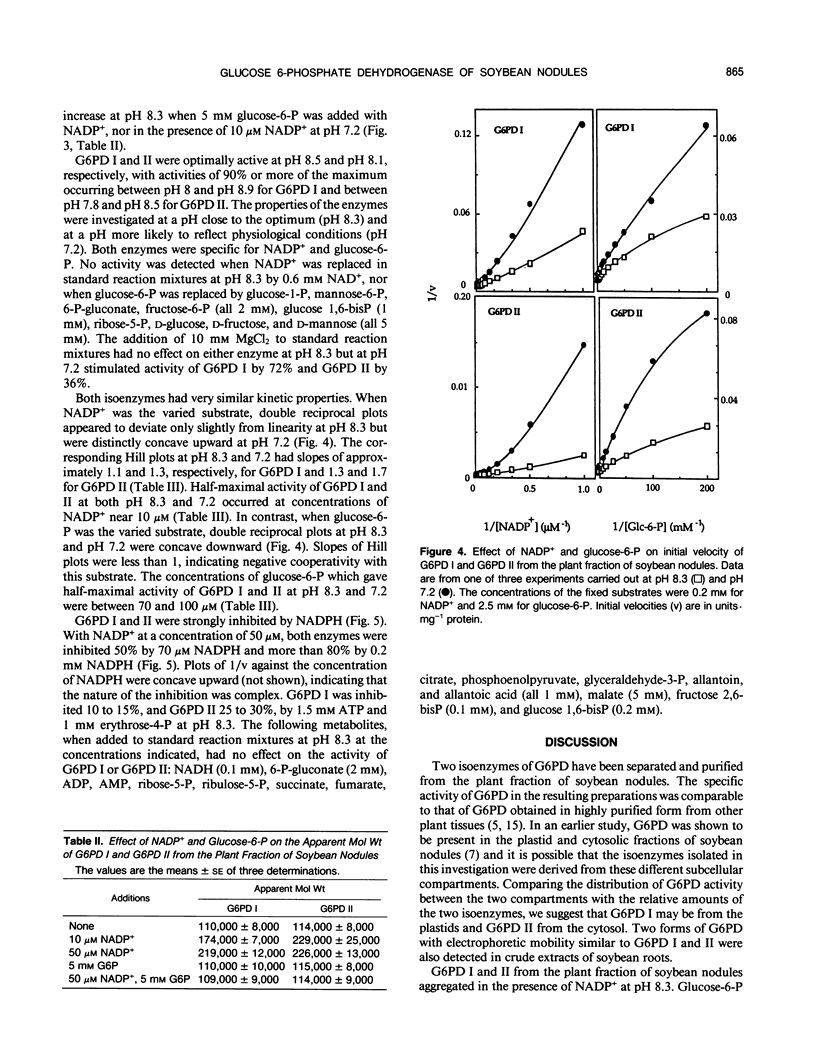
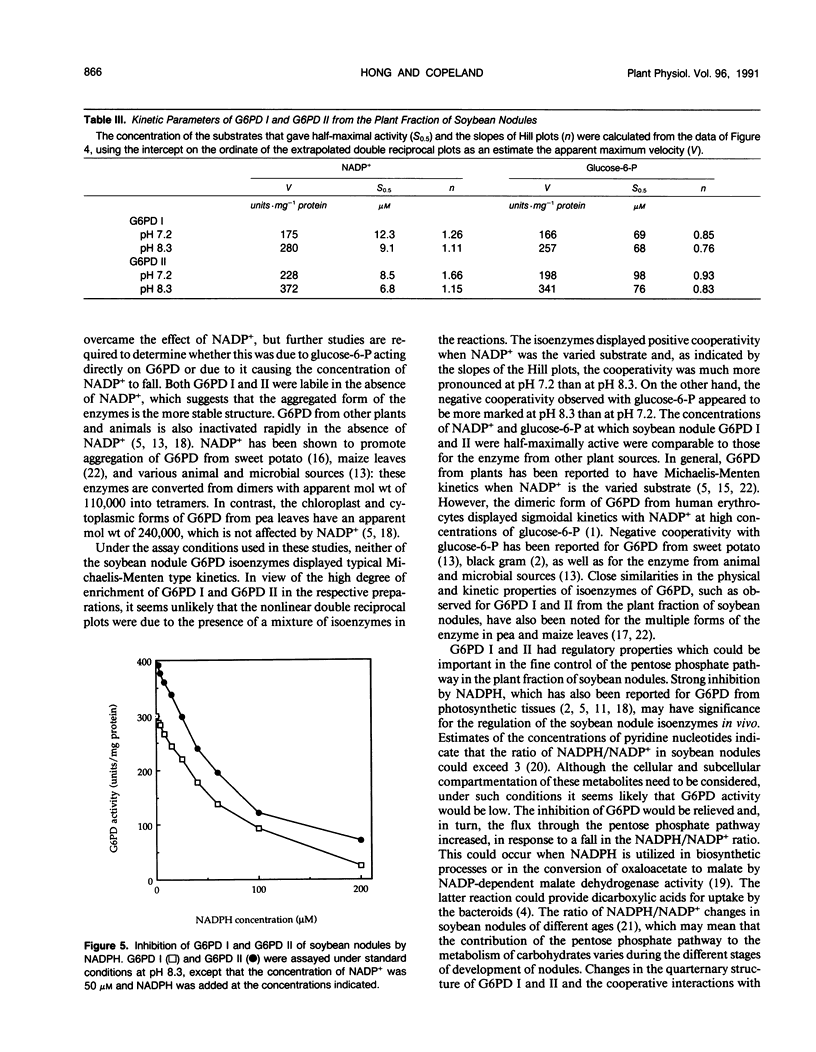
Two isoenzymes of glucose 6-phosphate dehydrogenase (EC 1.1.1.49) have been separated from the plant fraction of soybean (Glycine max L. Merr. cv Williams) nodules by a procedure involving (NH4)2SO4 gradient fractionation, gel chromatography, chromatofocusing, and affinity chromatography. The isoenzymes, which have been termed glucose 6-phosphate dehydrogenases I and II, were specific for NADP+ and glucose 6-phosphate and had optimum activity at pH 8.5 and pH 8.1, respectively. Both isoenzymes were labile in the absence of NADP+. The apparent molecular weight of glucose 6-phosphate dehydrogenases I and II at pH 8.3 was estimated by gel chromatography to be approximately 110,000 in the absence of NADP+ and double this size in the presence of NADP+. The apparent molecular weight did not increase when glucose 6-phosphate was added with NADP+ at pH 8.3. Both isoenzymes had very similar kinetic properties, displaying positive cooperativity in their interaction with NADP+ and negative cooperativity with glucose 6-phosphate. The isoenzymes had half-maximal activity at approximately 10 micromolar NADP+ and 70 to 100 micromolar glucose 6-phosphate. NADPH was a potent inhibitor of both of the soybean nodule glucose 6-phosphate dehydrogenases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adediran S. A. Sigmoid kinetics of human erythrocyte glucose-6-phosphate dehydrogenase. Arch Biochem Biophys. 1988 Apr;262(1):354–359. doi: 10.1016/0003-9861(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Fickenscher K., Scheibe R. Purification and properties of the cytoplasmic glucose-6-phosphate dehydrogenase from pea leaves. Arch Biochem Biophys. 1986 Jun;247(2):393–402. doi: 10.1016/0003-9861(86)90598-9. [DOI] [PubMed] [Google Scholar]
- King T. P. Separation of proteins by ammonium sulfate gradient solubilization. Biochemistry. 1972 Feb 1;11(3):367–371. doi: 10.1021/bi00753a010. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: II. Studies with C-labeled Glucose, the Pathway of Glucose Catabolism, and the Effects of Some Treatments That Inhibit Nitrogen Fixation. Plant Physiol. 1979 Mar;63(3):450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendzian K., Bassham J. A. Regulation of glucose-6-phosphate dehydrogenase in spinach chloroplasts by ribulose 1,5-diphosphate and NADPH/NADP+ ratios. Biochim Biophys Acta. 1975 Aug 11;396(2):260–275. doi: 10.1016/0005-2728(75)90040-7. [DOI] [PubMed] [Google Scholar]
- Levy H. R. Glucose-6-phosphate dehydrogenases. Adv Enzymol Relat Areas Mol Biol. 1979;48:97–192. doi: 10.1002/9780470122938.ch3. [DOI] [PubMed] [Google Scholar]
- Morell M., Copeland L. Enzymes of sucrose breakdown in soybean nodules: alkaline invertase. Plant Physiol. 1984 Apr;74(4):1030–1034. doi: 10.1104/pp.74.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Valenti V., Stanghellini M. A., Pupillo P. Glucose 6-phosphate dehydrogenase isozymes of maize leaves : some comparative properties. Plant Physiol. 1984 Jul;75(3):521–526. doi: 10.1104/pp.75.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. P., Evans H. J. Poly-beta-hydroxybutyrate Utilization by Soybean (Glycine max Merr.) Nodules and Assessment of Its Role in Maintenance of Nitrogenase Activity. Plant Physiol. 1971 Jun;47(6):750–755. doi: 10.1104/pp.47.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]