Abstract
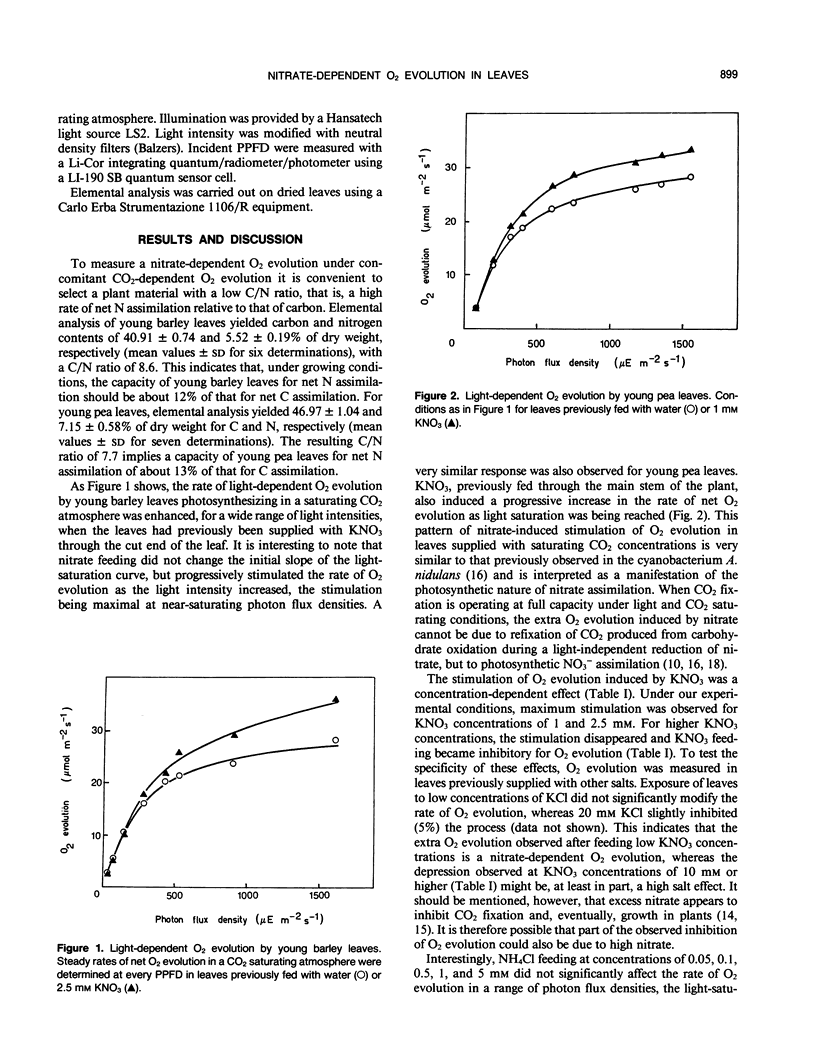
Evolution of O2 by illuminated intact detached leaves from barley (Hordeum vulgare L. cv Athos) and pea (Pisum sativum L. cv Lincoln) in a CO2-saturating atmosphere was enhanced when KNO3 (1-2.5 millimolar) had been previously supplied through the transpiration stream. The extra O2 evolution observed after feeding KNO3 increased with the light intensity, being maximal at near saturating photon flux densities and resulting in no changes in the initial slope of the O2 versus light-intensity curve. No stimulation of O2 evolution was otherwise observed after feeding KCl or NH4Cl. The data indicate that nitrate assimilation uses photosynthetically generated reductant and stimulates the rate of non-cyclic electron flow by acting as a second electron-accepting assimilatory process in addition to CO2 fixation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom A. J., Caldwell R. M., Finazzo J., Warner R. L., Weissbart J. Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol. 1989 Sep;91(1):352–356. doi: 10.1104/pp.91.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delieu T. J., Walker D. A. Simultaneous measurement of oxygen evolution and chlorophyll fluorescence from leaf pieces. Plant Physiol. 1983 Nov;73(3):534–541. doi: 10.1104/pp.73.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace G. M., Volk R. J., Jackson W. A. Nitrate Reduction in Response to CO(2)-Limited Photosynthesis : Relationship to Carbohydrate Supply and Nitrate Reductase Activity in Maize Seedlings. Plant Physiol. 1990 Feb;92(2):286–292. doi: 10.1104/pp.92.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K. Malate and Dihydroxyacetone Phosphate-dependent Nitrate Reduction in Spinach Leaf Protoplasts. Plant Physiol. 1978 Aug;62(2):220–223. doi: 10.1104/pp.62.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J. M., Lara C. Photosynthetic Assimilation of NO(3) by Intact Cells of the Cyanobacterium Anacystis nidulans: Influence of NO(3) and NH(4) Assimilation on CO(2) Fixation. Plant Physiol. 1987 Jan;83(1):208–212. doi: 10.1104/pp.83.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J. M., Lara C., Sivak M. N. Changes in Net O(2) Exchange Induced by Inorganic Nitrogen in the Blue-Green Alga Anacystis nidulans. Plant Physiol. 1989 Sep;91(1):28–30. doi: 10.1104/pp.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN NIEL C. B., ALLEN M. B., WRIGHT B. E. On the photochemical reduction of nitrate by algae. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):67–74. doi: 10.1016/0006-3002(53)90124-3. [DOI] [PubMed] [Google Scholar]


