Abstract
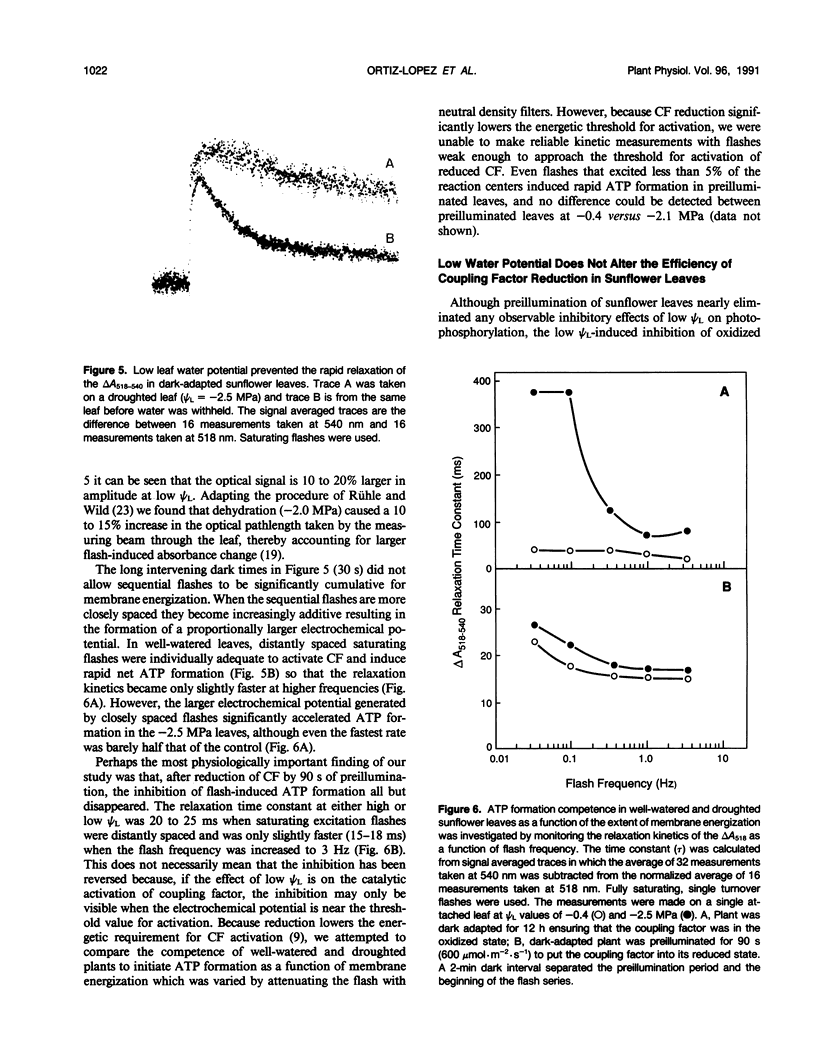
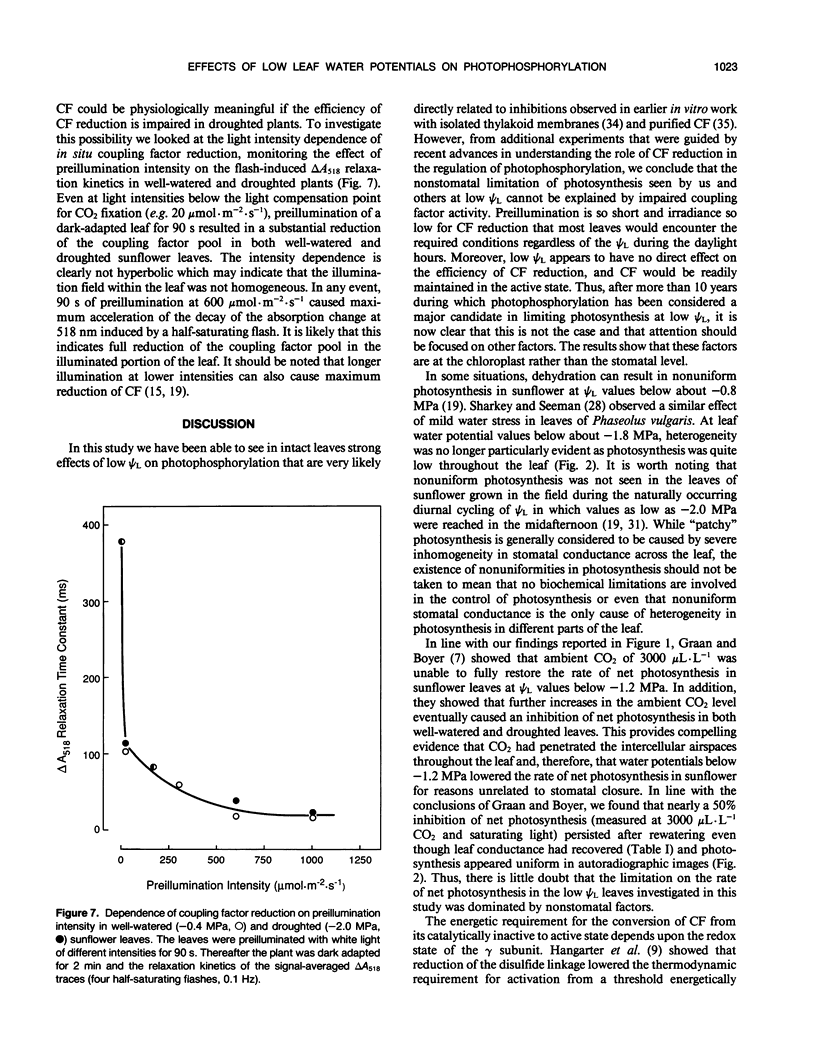
The in situ response of photophosphorylation and coupling factor activity to low leaf water potential (ψL) was investigated using kinetic spectroscopy to measure the flash-induced electrochromic absorption change in attached sunflower (Helianthus annuus L. cv IS894) leaves. The electrochromic change is caused by the formation of an electric potential across the thylakoid membrane associated with proton uptake. Since depolarization of the thylakoid membrane following flash excitation is normally dominated by proton efflux through the coupling factor during ATP formation, this measurement can provide direct information about the catalytic activity of the coupling factor. Under low ψL conditions in which a clear nonstomatal limitation of net photosynthesis could be demonstrated, we found a strong inhibition of coupling factor activity in dark-adapted leaves which was probably caused by an increase in the energetic threshold for the activation of the enzyme at low ψL. While this result supported earlier in vitro findings, we further discovered that the light-dependent reduction of coupling factor reversed any observable effect of low ψL on the energetics of activation or on photophosphorylation competence. Furthermore, coupling factor was reduced, even in severely droughted sunflower, almost immediately upon illumination. Based on these measurements, we conclude that the nonstomatal limitation of photosynthesis observed by us and others in droughted plants cannot be explained by impaired coupling factor activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz G. A., Gibbs M. Effect of osmotic stress on photosynthesis studied with the isolated spinach chloroplast : generation and use of reducing power. Plant Physiol. 1982 Oct;70(4):1143–1148. doi: 10.1104/pp.70.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R. P., Grandoni P., Ort D. R. The effects of chloroplast coupling factor reduction on the energetics of activation and on the energetics and efficiency of ATP formation. J Biol Chem. 1987 Oct 5;262(28):13513–13519. [PubMed] [Google Scholar]
- Ketcham S. R., Davenport J. W., Warncke K., McCarty R. E. Role of the gamma subunit of chloroplast coupling factor 1 in the light-dependent activation of photophosphorylation and ATPase activity by dithiothreitol. J Biol Chem. 1984 Jun 10;259(11):7286–7293. [PubMed] [Google Scholar]
- Plaut Z. Inhibition of photosynthetic carbon dioxide fixation in isolated spinach chloroplasts exposed to reduced osmotic potentials. Plant Physiol. 1971 Nov;48(5):591–595. doi: 10.1104/pp.48.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, McCarty R. E. Effects of adenine nucleotides and of photophosphorylation on H+ uptake and the magnitude of the H+ gradient in illuminated chloroplasts. J Biol Chem. 1974 Oct 10;249(19):6250–6254. [PubMed] [Google Scholar]
- Rebeille F., Hatch M. D. Regulation of NADP-malate dehydrogenase in C4 plants: relationship among enzyme activity, NADPH to NADP ratios, and thioredoxin redox states in intact maize mesophyll chloroplasts. Arch Biochem Biophys. 1986 Aug 15;249(1):171–179. doi: 10.1016/0003-9861(86)90572-2. [DOI] [PubMed] [Google Scholar]
- Scheibe R., Geissler A., Fickenscher K. Chloroplast glucose-6-phosphate dehydrogenase: Km shift upon light modulation and reduction. Arch Biochem Biophys. 1989 Oct;274(1):290–297. doi: 10.1016/0003-9861(89)90441-4. [DOI] [PubMed] [Google Scholar]
- Shahak Y. Activation and Deactivation of H-ATPase in Intact Chloroplasts. Plant Physiol. 1982 Jul;70(1):87–91. doi: 10.1104/pp.70.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Seemann J. R. Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiol. 1989 Apr;89(4):1060–1065. doi: 10.1104/pp.89.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejos R. H., Arana J. L., Ravizzini R. A. Changes in activity and structure of the chloroplast proton ATPase induced by illumination of spinach leaves. J Biol Chem. 1983 Jun 25;258(12):7317–7321. [PubMed] [Google Scholar]
- Wise R. R., Ort D. R. Photophosphorylation after chilling in the light : effects on membrane energization and coupling factor activity. Plant Physiol. 1989 Jun;90(2):657–664. doi: 10.1104/pp.90.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979 Mar 14;505(3-4):355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- Younis H. M., Boyer J. S., Govindjee Conformation and activity of chloroplast coupling factor exposed to low chemical potential of water in cells. Biochim Biophys Acta. 1979 Nov 8;548(2):328–340. doi: 10.1016/0005-2728(79)90139-7. [DOI] [PubMed] [Google Scholar]