Abstract
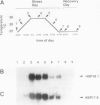
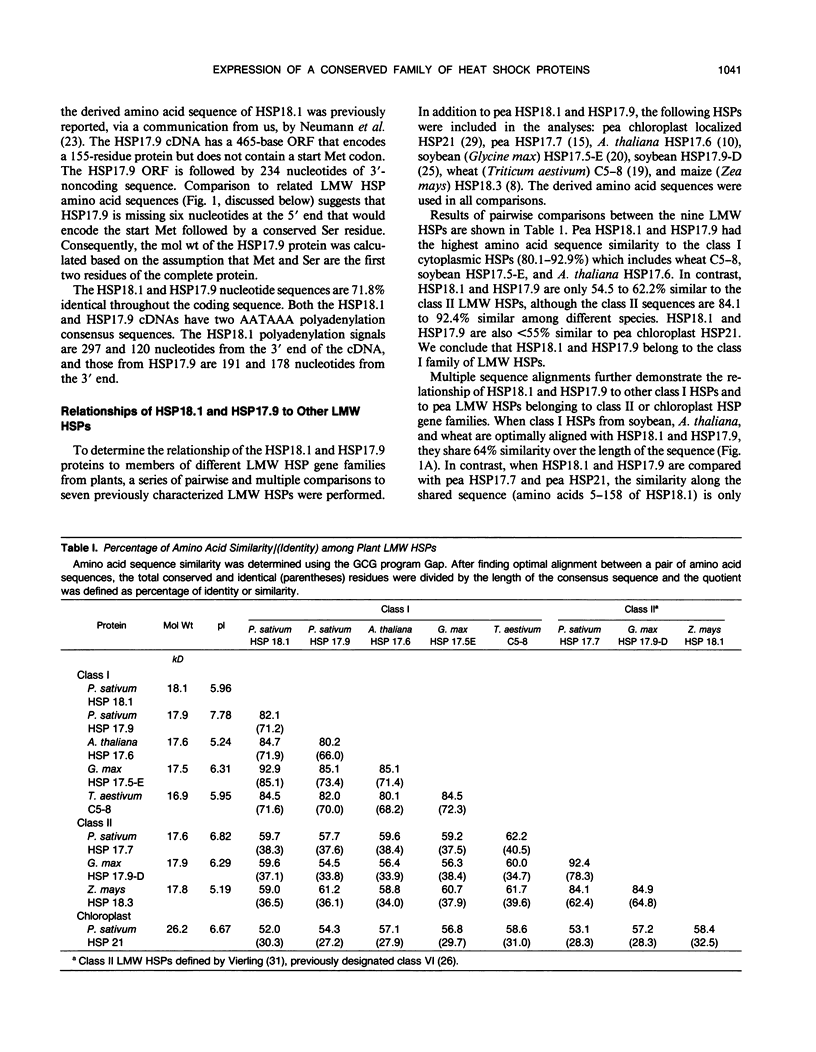
Plants synthesize several families of low molecular weight (LMW) heat shock proteins (HSPs) in response to elevated temperatures. We have characterized two cDNAs, HSP18.1 and HSP17.9, that encode members of the class I family of LMW HSPs from pea (Pisum sativum). In addition, we investigated the expression of these HSPs at the mRNA and protein levels during heat stress and recovery. HSP18.1 and HSP17.9 are 82.1% identical at the amino acid level and are 80.8 to 92.9% identical to class I LMW HSPs of other angiosperms. Heat stress experiments were performed using intact seedlings subjected to a gradual temperature increase and held at a maximum temperature of 30 to 42 degrees Celsius for 4 hours. HSP18.1 and HSP17.9 mRNA levels peaked at the beginning of the maximum temperature period and declined rapidly after the stress period. Antiserum against a HSP18.1 fusion protein recognized both HSP18.1 and HSP17.9 but not members of other families of LMW HSPs. The accumulation of HSP18.1-immunodetected protein was proportional to the severity of the heat stress, and the protein had a half-life of 37.7 ± 8 hours. The long half-life of these proteins supports the hypothesis that they are involved in establishing thermotolerance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. J., Hatfield J. L., Klein R. R., Mullet J. E. Accumulation of heat shock proteins in field-grown cotton. Plant Physiol. 1985 Jun;78(2):394–398. doi: 10.1104/pp.78.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Lauzon L. M., DeRocher A. E., Vierling E. Accumulation, stability, and localization of a major chloroplast heat-shock protein. J Cell Biol. 1990 Jun;110(6):1873–1883. doi: 10.1083/jcb.110.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Goping I. S., Frappier J. R., Walden D. B., Atkinson B. G. Sequence, identification and characterization of cDNAs encoding two different members of the 18 kDa heat shock family of Zea mays L. Plant Mol Biol. 1991 Apr;16(4):699–711. doi: 10.1007/BF00023434. [DOI] [PubMed] [Google Scholar]
- Helm K. W., Petersen N. S., Abernethy R. H. Heat shock response of germinating embryos of wheat : effects of imbibition time and seed vigor. Plant Physiol. 1989 Jun;90(2):598–605. doi: 10.1104/pp.90.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm K. W., Vierling E. An Arabidopsis thaliana cDNA clone encoding a low molecular weight heat shock protein. Nucleic Acids Res. 1989 Oct 11;17(19):7995–7995. doi: 10.1093/nar/17.19.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Kimpel J. A., Key J. L. Presence of Heat Shock mRNAs in Field Crown Soybeans. Plant Physiol. 1985 Nov;79(3):672–678. doi: 10.1104/pp.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel J. A., Nagao R. T., Goekjian V., Key J. L. Regulation of the heat shock response in soybean seedlings. Plant Physiol. 1990 Nov;94(3):988–995. doi: 10.1104/pp.94.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon L. M., Helm K. W., Vierling E. A cDNA clone from Pisum sativum encoding a low molecular weight heat shock protein. Nucleic Acids Res. 1990 Jul 25;18(14):4274–4274. doi: 10.1093/nar/18.14.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mansfield M. A., Key J. L. Synthesis of the low molecular weight heat shock proteins in plants. Plant Physiol. 1987 Aug;84(4):1007–1017. doi: 10.1104/pp.84.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain E. F., Spiker S. A wheat cDNA clone which is homologous to the 17 kd heat-shock protein gene family of soybean. Nucleic Acids Res. 1989 Feb 25;17(4):1764–1764. doi: 10.1093/nar/17.4.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R. T., Czarnecka E., Gurley W. B., Schöffl F., Key J. L. Genes for low-molecular-weight heat shock proteins of soybeans: sequence analysis of a multigene family. Mol Cell Biol. 1985 Dec;5(12):3417–3428. doi: 10.1128/mcb.5.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Nover L., Parthier B., Rieger R., Scharf K. D., Wollgiehn R., zur Nieden U. Heat shock and other stress response systems of plants. Results Probl Cell Differ. 1989;16:1–155. [PubMed] [Google Scholar]
- Nieto-Sotelo J., Vierling E., Ho T. H. Cloning, sequence analysis, and expression of a cDNA encoding a plastid-localized heat shock protein in maize. Plant Physiol. 1990 Aug;93(4):1321–1328. doi: 10.1104/pp.93.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke E., Baumann G., Schöffl F. Nucleotide sequence analysis of soybean small heat shock protein genes belonging to two different multigene families. J Mol Biol. 1988 Feb 20;199(4):549–557. doi: 10.1016/0022-2836(88)90300-2. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Harris L. M., Chen Q. The major low-molecular-weight heat shock protein in chloroplasts shows antigenic conservation among diverse higher plant species. Mol Cell Biol. 1989 Feb;9(2):461–468. doi: 10.1128/mcb.9.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Mishkind M. L., Schmidt G. W., Key J. L. Specific heat shock proteins are transported into chloroplasts. Proc Natl Acad Sci U S A. 1986 Jan;83(2):361–365. doi: 10.1073/pnas.83.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Nagao R. T., DeRocher A. E., Harris L. M. A heat shock protein localized to chloroplasts is a member of a eukaryotic superfamily of heat shock proteins. EMBO J. 1988 Mar;7(3):575–581. doi: 10.1002/j.1460-2075.1988.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]