Abstract
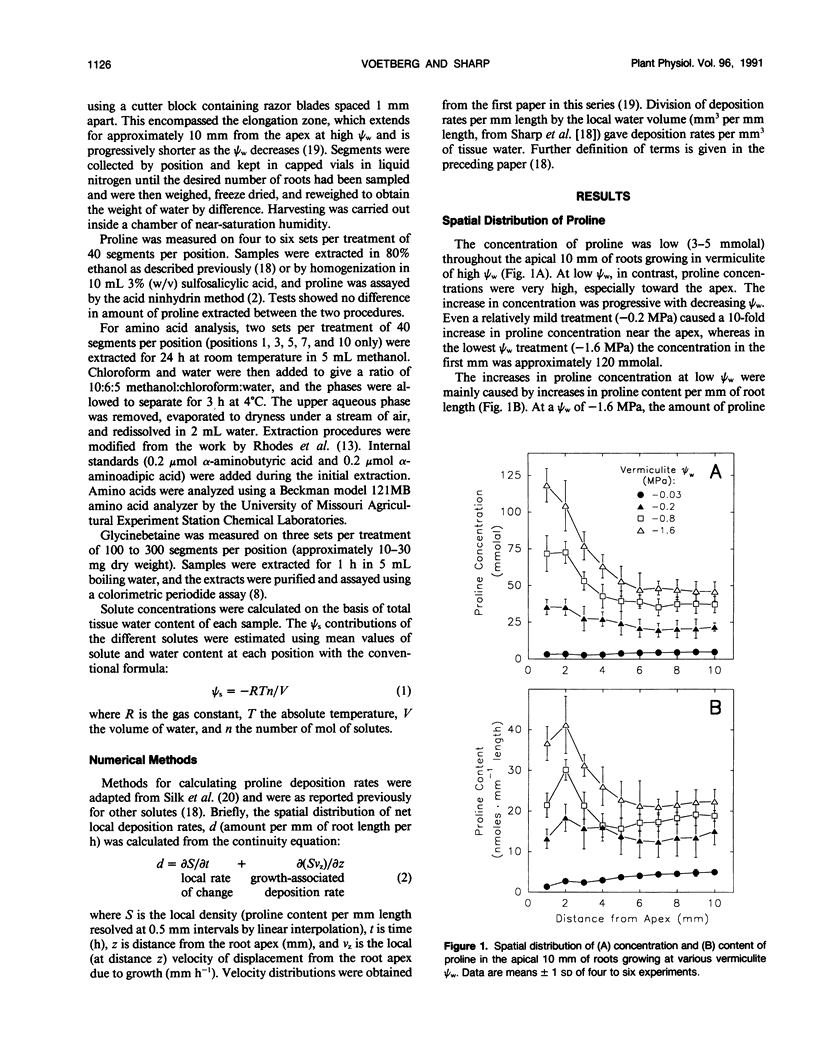
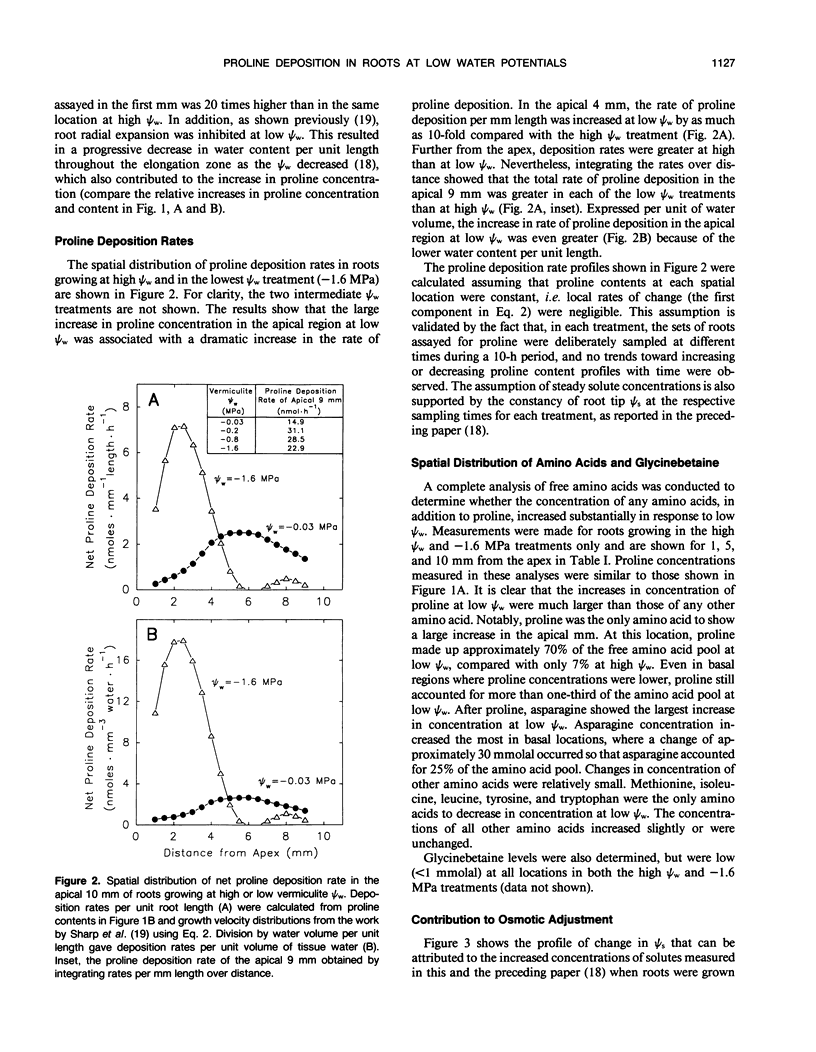
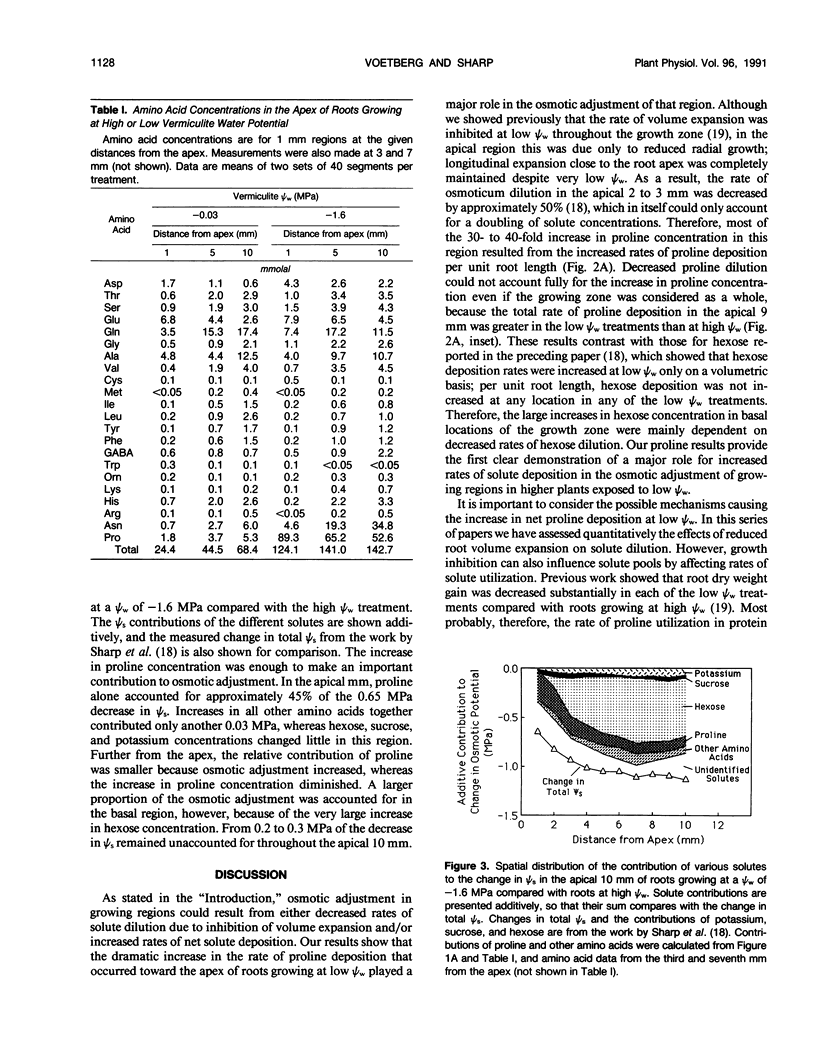
Seedlings of maize (Zea mays L. cv WF9 × Mo 17) growing at low water potentials in vermiculite contained greatly increased proline concentrations in the primary root growth zone. Proline levels were particularly high toward the apex, where elongation rates have been shown to be completely maintained over a wide range of water potentials. Proline concentration increased even in quite mild treatments and reached 120 millimolal in the apical millimeter of roots growing at a water potential of −1.6 megapascal. This accounted for almost half of the osmotic adjustment in this region. Increases in concentration of other amino acids and glycinebetaine were comparatively small. We have assessed the relative contributions of increased rates of proline deposition and decreased tissue volume expansion to the increases in proline concentration. Proline content profiles were combined with published growth velocity distributions to calculate net proline deposition rate profiles using the continuity equation. At low water potential, proline deposition per unit length increased by up to 10-fold in the apical region of the growth zone compared to roots at high water potential. This response accounted for most of the increase in proline concentration in this region. The results suggest that osmotic adjustment due to increased proline deposition plays an important role in the maintenance of root elongation at low water potentials.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggess S. F., Stewart C. R. Effect of water stress on proline synthesis from radioactive precursors. Plant Physiol. 1976 Sep;58(3):398–401. doi: 10.1104/pp.58.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk D. G., Rich P. J., Rhodes D. Genotypic Variation for Glycinebetaine among Public Inbreds of Maize. Plant Physiol. 1989 Nov;91(3):1122–1125. doi: 10.1104/pp.91.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greacen E. L., Oh J. S. Physics of root growth. Nat New Biol. 1972 Jan 5;235(53):24–25. doi: 10.1038/newbio235024a0. [DOI] [PubMed] [Google Scholar]
- Oaks A. Transport of amino acids to the maize root. Plant Physiol. 1966 Jan;41(1):173–180. doi: 10.1104/pp.41.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci P. Involvement of Cl in the Increase in Proline Induced by ABA and Stimulated by Potassium Chloride in Barley Leaf Segments. Plant Physiol. 1989 Apr;89(4):1226–1230. doi: 10.1104/pp.89.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Handa S., Bressan R. A. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986 Dec;82(4):890–903. doi: 10.1104/pp.82.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab I. N., Sharp R. E., Pritchard J., Voetberg G. S. Increased endogenous abscisic Acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 1990 Aug;93(4):1329–1336. doi: 10.1104/pp.93.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R. E., Hsiao T. C., Silk W. K. Growth of the Maize Primary Root at Low Water Potentials : II. Role of Growth and Deposition of Hexose and Potassium in Osmotic Adjustment. Plant Physiol. 1990 Aug;93(4):1337–1346. doi: 10.1104/pp.93.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R. E., Silk W. K., Hsiao T. C. Growth of the maize primary root at low water potentials : I. Spatial distribution of expansive growth. Plant Physiol. 1988 May;87(1):50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Walker R. C., Labavitch J. Uronide Deposition Rates in the Primary Root of Zea mays. Plant Physiol. 1984 Mar;74(3):721–726. doi: 10.1104/pp.74.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spollen W. G., Sharp R. E. Spatial distribution of turgor and root growth at low water potentials. Plant Physiol. 1991 Jun;96(2):438–443. doi: 10.1104/pp.96.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Inhibition of proline oxidation by water stress. Plant Physiol. 1977 May;59(5):930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]


