Abstract
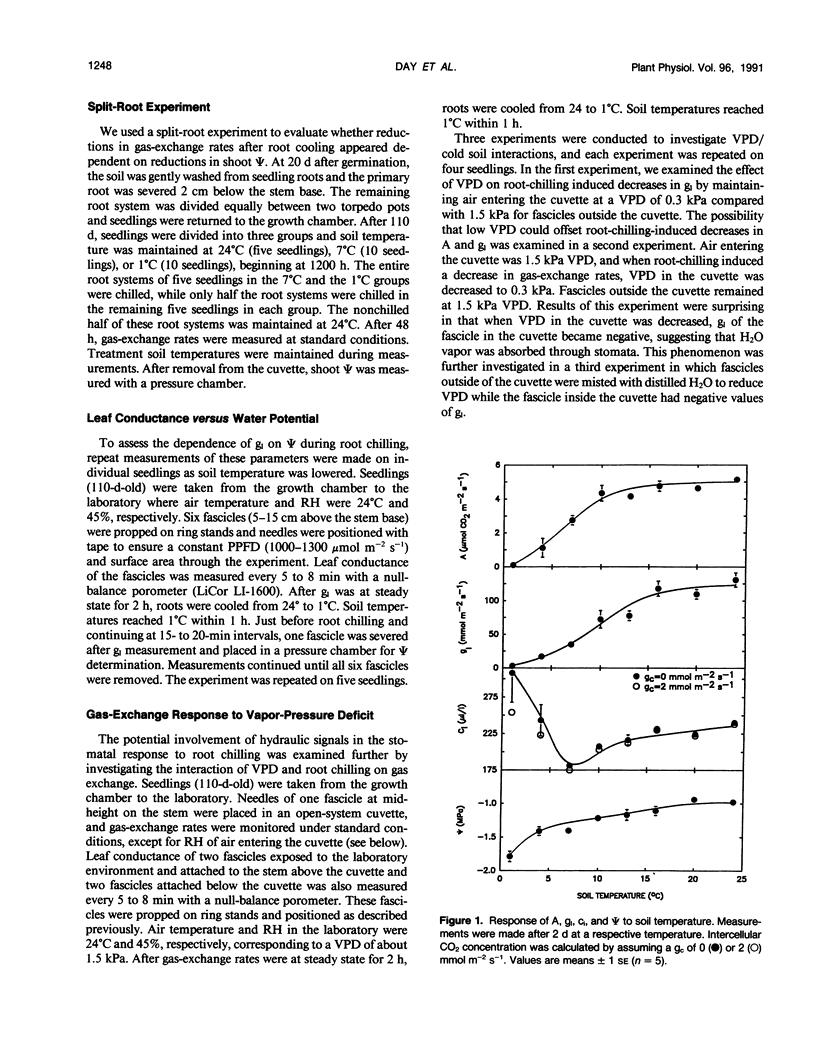
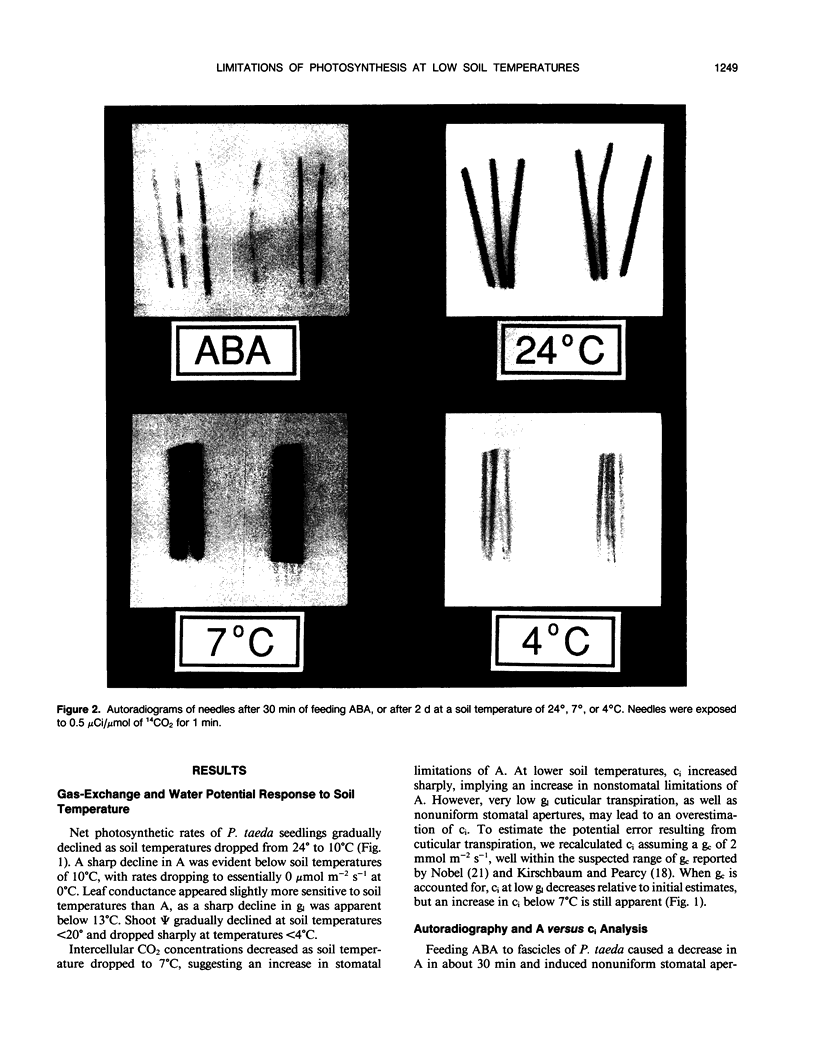
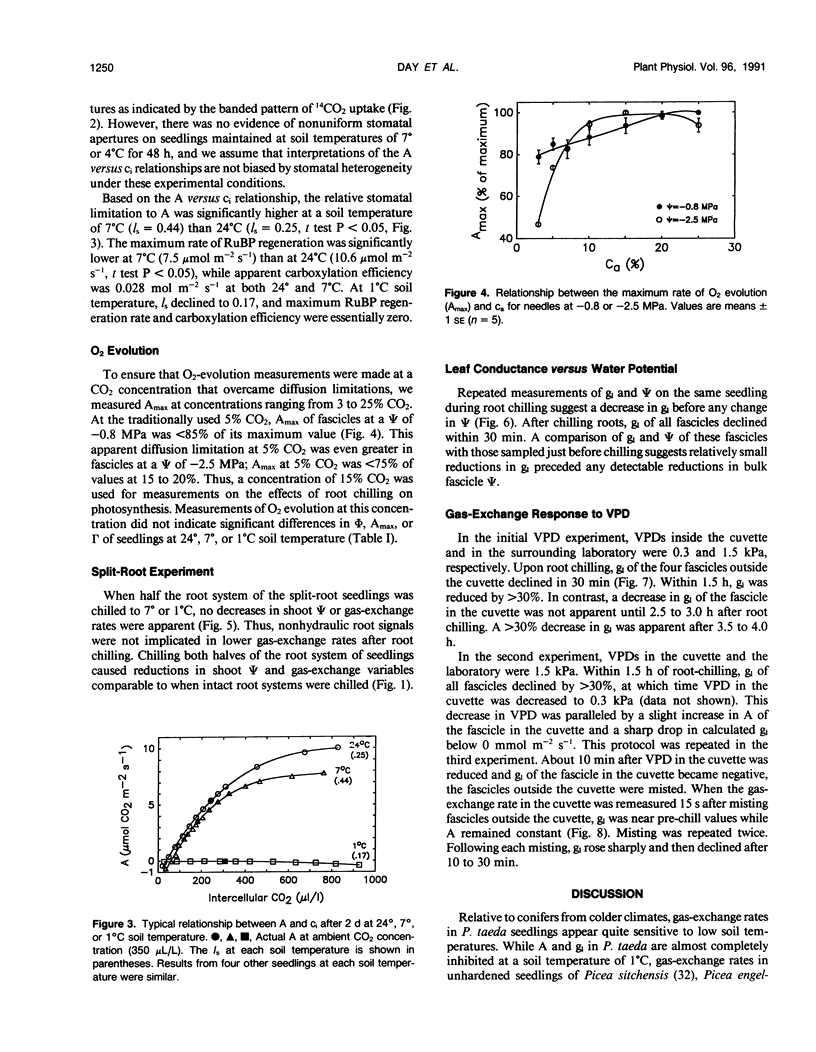
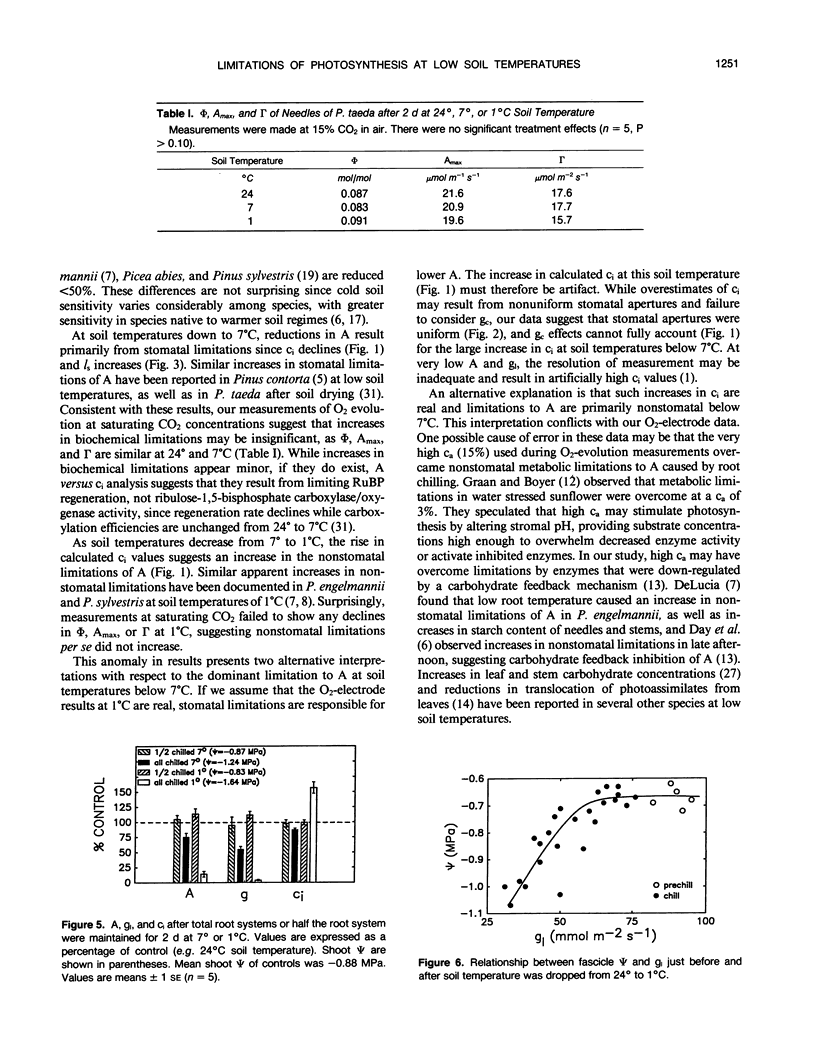
The relative importance of stomatal and nonstomatal limitations to net photosynthesis (A) and possible signals responsible for stomatal limitations were investigated in unhardened Pinus taeda seedlings at low soil temperatures. After 2 days at soil temperatures between 13 and 7°C, A was reduced by 20 to 50%, respectively. The reduction in A at these moderate root-chilling conditions appeared to be the result of stomatal limitations, based on the decrease in intercellular CO2 concentrations (ci). This conclusion was supported by A versus ci analysis and measurements of O2 evolution at saturating CO2, which suggested increases in stomatal but not biochemical limitations at these soil temperatures. Nonuniform stomatal apertures, which were demonstrated with abscisic acid, were not apparent 2 days after root chilling, and results of our A versus ci analysis appear valid. Bulk shoot water potential (ψ) declined as soil temperature dropped below 16°C. When half the root system of seedlings was chilled, shoot ψ and gas-exchange rates did not decline. Thus, nonhydraulic root-shoot signals were not implicated in stomatal limitations. The initial decrease in leaf conductance to water vapor after root chilling appeared to precede any detectable decrease in bulk fascicle ψ, but may be in response to a decrease in turgor of epidermal cells. These reductions in leaf conductance to water vapor, which occurred within 30 minutes of root chilling, could be delayed and temporarily reversed by reducing the leaf-to-air vapor-pressure deficit, suggesting that hydraulic signals may be involved in initiating stomatal closure. By independently manipulating the leaf-to-air vapor-pressure deficit of individual fascicles, we could induce uptake of water vapor through stomata, suggesting that nonsaturated conditions occur in the intercellular airspaces. There was an anomaly in our results on seedlings maintained for 2 days at soil temperatures below 7°C. Lower A appeared primarily the result of nonstomatal limitations, based on large increases in calculated ci and A versus ci analysis. In contrast, measurements of O2 evolution at saturating CO2 concentrations implied nonstomatal limitations per se did not increase at these temperatures. One explanation for this paradox is that calculations of ci are unreliable at very low gas-exchange rates because of inadequate measurement resolution, and limitations of A are predominantly stomatal. An alternative interpretation is that increases in ci are real and the results from O2-evolution measurements are in error. The high CO2 concentration used in O2-evolution measurements (15%) may have overcome nonstomatal limitations by enzymes that were down-regulated by a feedback mechanism. In this scenario, carbohydrate feedback limitations may be responsible for nonstomatal reductions in A after 2 days at soil temperatures below 7°C.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delucia Evan H. Effect of low root temperature on net photosynthesis, stomatal conductance and carbohydrate concentration in Engelmann spruce (Picea engelmannii Parry ex Engelm.) seedlings. Tree Physiol. 1986 Dec;2(1_2_3):143–154. doi: 10.1093/treephys/2.1-2-3.143. [DOI] [PubMed] [Google Scholar]
- Hurewitz J., Janes H. W. Effect of altering the root-zone temperature on growth, translocation, carbon exchange rate, and leaf starch accumulation in the tomato. Plant Physiol. 1983 Sep;73(1):46–50. doi: 10.1104/pp.73.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum M. U., Pearcy R. W. Gas Exchange Analysis of the Relative Importance of Stomatal and Biochemical Factors in Photosynthetic Induction in Alocasia macrorrhiza. Plant Physiol. 1988 Mar;86(3):782–785. doi: 10.1104/pp.86.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer P. J. ROOT RESISTANCE AS A CAUSE OF DECREASED WATER ABSORPTION BY PLANTS AT LOW TEMPERATURES. Plant Physiol. 1940 Jan;15(1):63–79. doi: 10.1104/pp.15.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W. Responses of transpiration and hydraulic conductance to root temperature in nitrogen- and phosphorus-deficient cotton seedlings. Plant Physiol. 1990 Mar;92(3):855–857. doi: 10.1104/pp.92.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running S. W., Reid C. P. Soil Temperature Influences on Root Resistance of Pinus contorta Seedlings. Plant Physiol. 1980 Apr;65(4):635–640. doi: 10.1104/pp.65.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackel K. A., Brinckmann E. In Situ Measurement of Epidermal Cell Turgor, Leaf Water Potential, and Gas Exchange in Tradescantia virginiana L. Plant Physiol. 1985 May;78(1):66–70. doi: 10.1104/pp.78.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teskey R. O., Fites J. A., Samuelson L. J., Bongarten B. C. Stomatal and nonstomatal limitations to net photosynthesis in Pinus taeda L. under different environmental conditions. Tree Physiol. 1986 Dec;2(1_2_3):131–142. doi: 10.1093/treephys/2.1-2-3.131. [DOI] [PubMed] [Google Scholar]



