Abstract
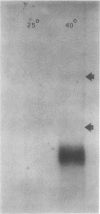
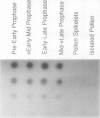
A maize (Zea mays L.) genomic clone (Zmempr 9′) was isolated on the basis of its homology to a meiotically expressed Lilium sequence. Radiolabeled probe made from the maize genomic clone detected complementary RNA at high fidelity. Furthermore, it hybridized to RNA isolated from staged (an interval that is coincident with meiotic prophase) maize tassel spikelets. Complimentary RNA was strongly (at least 50-fold) induced during heat shock of maize somatic tissue and appeared as a single size class in Northern blot hybridizations. Sequencing of the complete coding region of Zmempr 9′ confirmed the homology of the inferred amino acid sequence to other small heat shock proteins. Consensus sequences found in the flanking regions corresponded to the usual signals for initiation of RNA transcription, polyadenylate addition, and the induction of heat shock genes. The latter sequences conferred heat shock-specific transient expression in electroporated protoplasts when cloned into promoterless reporter gene plasmid constructs. Hybrid-selected translations revealed specific translation products ranging from 15 to 18 kilodaltons, providing evidence that this gene is a member of a related multigene family. We therefore conclude that this maize genomic DNA clone, recovered through its homology to clones for meiotic transcripts in lily, represents a genuine maize small heat shock protein gene.
Full text
PDF

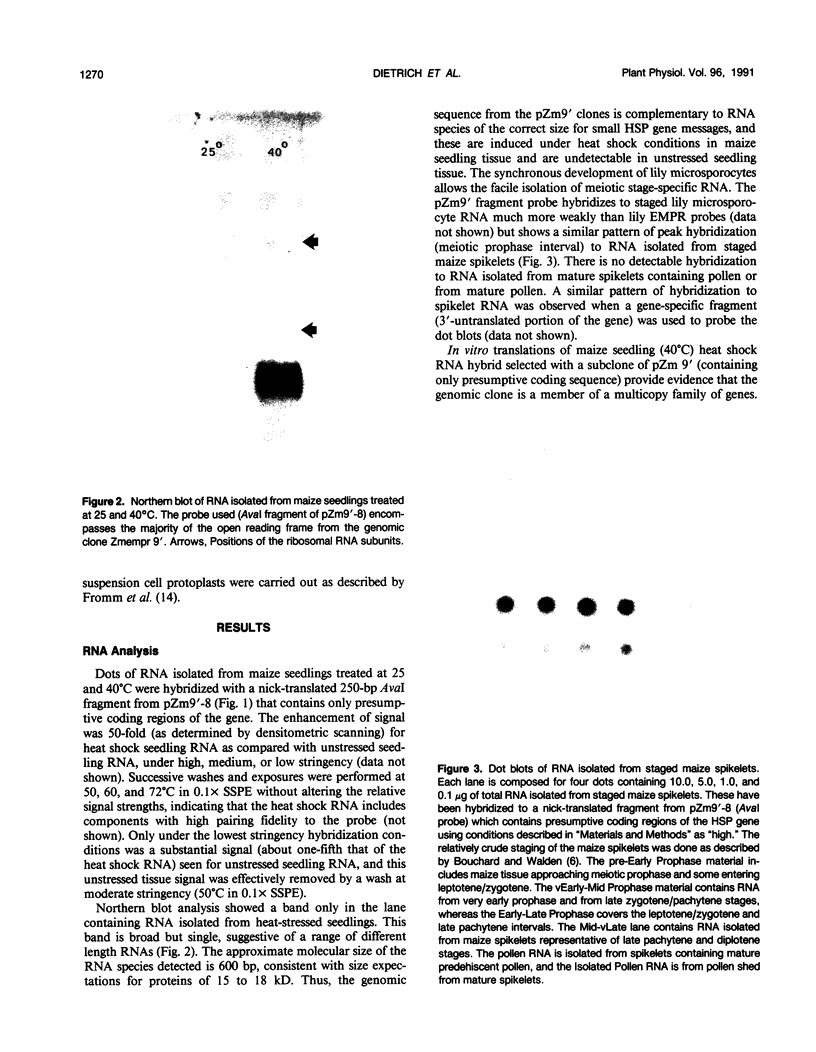






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainley W. M., Key J. L. Development of a heat shock inducible expression cassette for plants: characterization of parameters for its use in transient expression assays. Plant Mol Biol. 1990 Jun;14(6):949–967. doi: 10.1007/BF00019392. [DOI] [PubMed] [Google Scholar]
- Bonham-Smith P. C., Kapoor M., Bewley J. D. Establishment of thermotolerance in maize by exposure to stresses other than a heat shock does not require heat shock protein synthesis. Plant Physiol. 1987 Oct;85(2):575–580. doi: 10.1104/pp.85.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard R. A. Characterization of expressed meiotic prophase repeat transcript clones of Lilium: meiosis-specific expression, relatedness, and affinities to small heat shock protein genes. Genome. 1990 Feb;33(1):68–79. doi: 10.1139/g90-012. [DOI] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Heat Inducible Expression of a Chimeric Maize hsp70CAT Gene in Maize Protoplasts. Plant Physiol. 1988 Dec;88(4):965–968. doi: 10.1104/pp.88.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Meselson M. Separate regulatory elements for the heat-inducible and ovarian expression of the Drosophila hsp26 gene. Cell. 1985 Dec;43(3 Pt 2):737–746. doi: 10.1016/0092-8674(85)90247-8. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecka E., Gurley W. B., Nagao R. T., Mosquera L. A., Key J. L. DNA sequence and transcript mapping of a soybean gene encoding a small heat shock protein. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3726–3730. doi: 10.1073/pnas.82.11.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecka E., Key J. L., Gurley W. B. Regulatory domains of the Gmhsp17.5-E heat shock promoter of soybean. Mol Cell Biol. 1989 Aug;9(8):3457–3463. doi: 10.1128/mcb.9.8.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- McElwain E. F., Spiker S. A wheat cDNA clone which is homologous to the 17 kd heat-shock protein gene family of soybean. Nucleic Acids Res. 1989 Feb 25;17(4):1764–1764. doi: 10.1093/nar/17.4.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R., Schiller P., Amin J., Klapper H., Ananthan J., Voellmy R. Heat shock and ecdysterone activation of the Drosophila melanogaster hsp23 gene; a sequence element implied in developmental regulation. EMBO J. 1986 Jul;5(7):1667–1673. doi: 10.1002/j.1460-2075.1986.tb04410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Lindquist S. Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul. 1989 Nov;1(1):135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Raschke E., Baumann G., Schöffl F. Nucleotide sequence analysis of soybean small heat shock protein genes belonging to two different multigene families. J Mol Biol. 1988 Feb 20;199(4):549–557. doi: 10.1016/0022-2836(88)90300-2. [DOI] [PubMed] [Google Scholar]
- Rhodes C. A., Pierce D. A., Mettler I. J., Mascarenhas D., Detmer J. J. Genetically transformed maize plants from protoplasts. Science. 1988 Apr 8;240(4849):204–207. doi: 10.1126/science.2832947. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Xiao C. M., Mascarenhas J. P. High temperature-induced thermotolerance in pollen tubes of tradescantia and heat-shock proteins. Plant Physiol. 1985 Aug;78(4):887–890. doi: 10.1104/pp.78.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. Germline transformation used to define key features of heat-shock response elements. Science. 1988 Mar 4;239(4844):1139–1142. doi: 10.1126/science.3125608. [DOI] [PubMed] [Google Scholar]
- Young S. L., Jackson A. E., Puett D., Melner M. H. Detection of chloramphenicol acetyl transferase activity in transfected cells: a rapid and sensitive HPLC-based method. DNA. 1985 Dec;4(6):469–475. doi: 10.1089/dna.1985.4.469. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]