Abstract
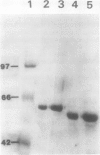
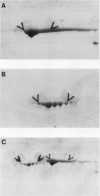
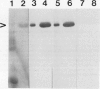
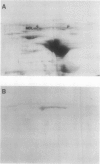
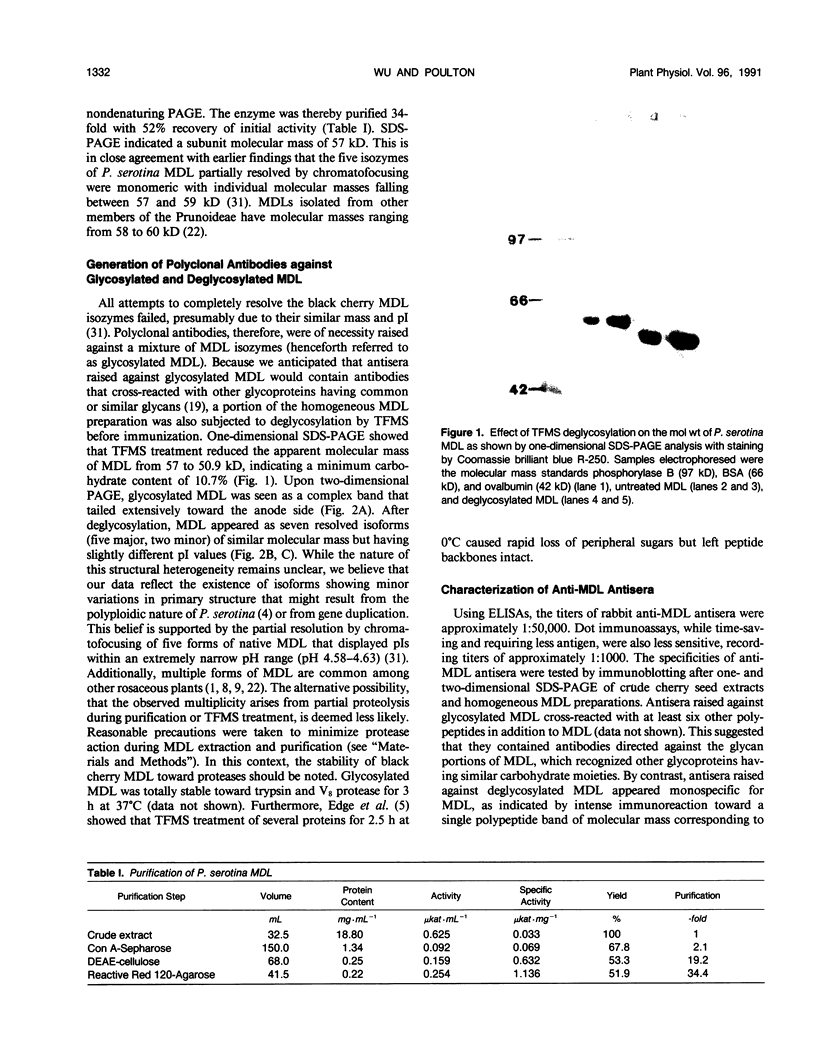
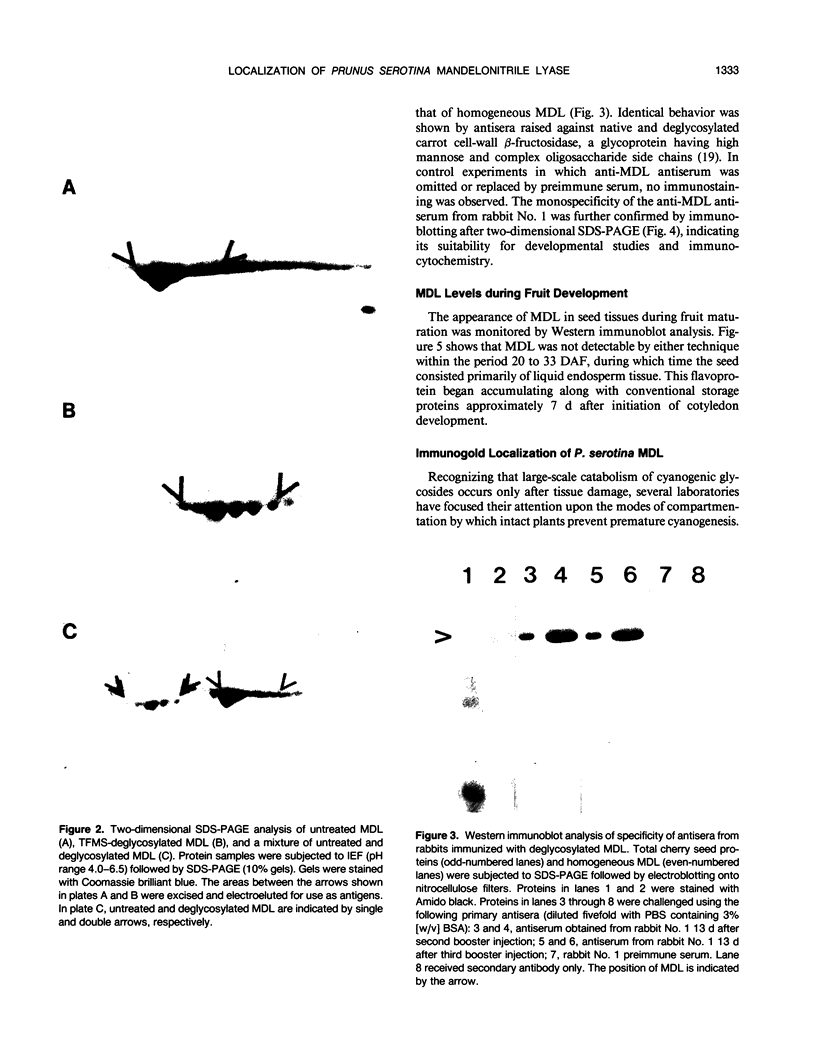
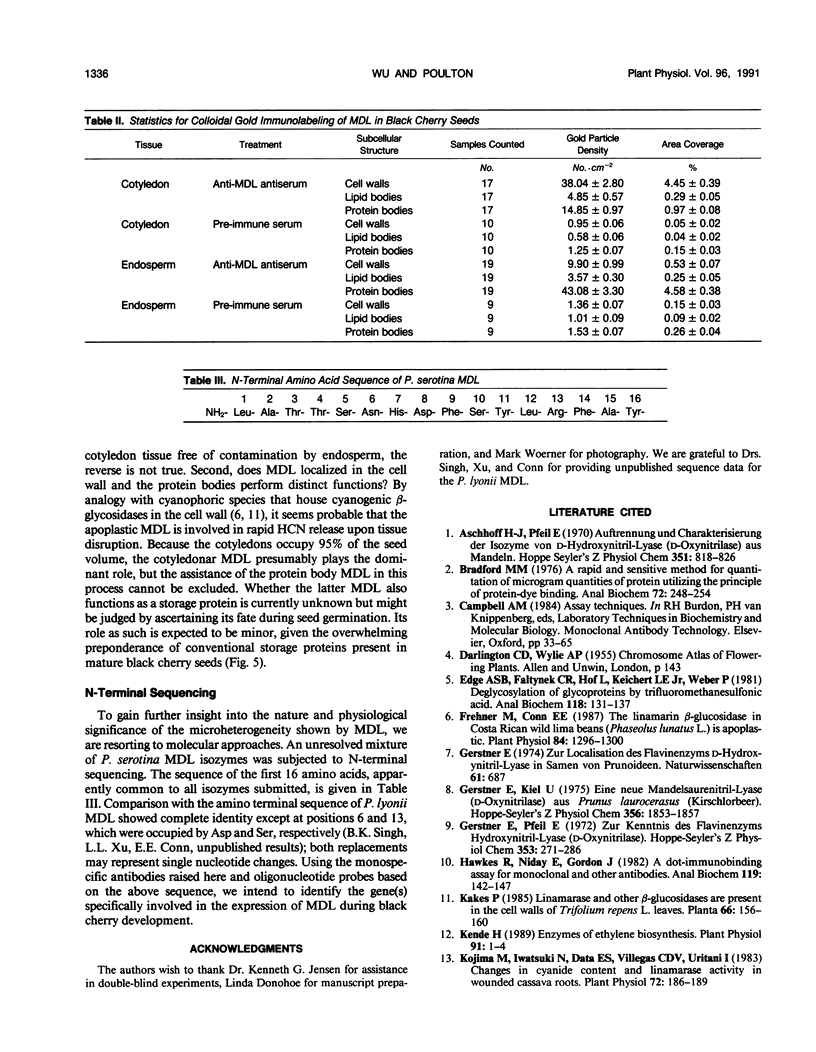
Mandelonitrile lyase (MDL, EC 4.1.2.10), which catalyzes the reversible dissociation of (R)-(+)-mandelonitrile to benzaldehyde and hydrogen cyanide, was purified to apparent homogeneity from mature black cherry (Prunus serotina Ehrh.) seeds by conventional protein purification techniques. This flavoprotein is monomeric with a subunit molecular mass of 57 kilodaltons. Glycoprotein character was shown by its binding to the affinity matrix concanavalin A-Sepharose 4B with subsequent elution by α-methyl-d-glucoside. Upon chemical deglycosylation by trifluoromethanesulfonic acid, the molecular mass was reduced to 50.9 kilodaltons. Two-dimensional gel analysis of deglycosylated MDL revealed the presence of several subunit isoforms of similar molecular mass but differing slightly in isoelectric point. Polyclonal antibodies were raised in New Zealand white rabbits against deglycosylated and untreated MDL. Antibody titers were determined by enzyme linked immunosorbent and dot immunobinding assays, while their specificities were assessed by Western immunoblot analysis. Antibodies raised against untreated lyase recognized several proteins in addition to MDL. In contrast, antisera raised against deglycosylated MDL were monospecific and were utilized for developmental and immunocytochemical localization studies. SDS-PAGE and immunoblotting analysis of seed proteins during fruit maturation showed that MDL first appeared in seeds shortly after cotyledons began development. In cotyledon cells of mature seeds, MDL was localized primarily in the cell wall with lesser amounts in the protein bodies, whereas in endosperm cells, this labeling pattern was reversed. N-terminal sequence data was gathered for future molecular approaches to the question of MDL microheterogeneity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschhoff H. J., Pfeil E. Auftrennung und Charakterisierung der Isoenzyme von D-Hydroxynitril-Lyase (D-Oxynitrilase) aus Mandeln. Hoppe Seylers Z Physiol Chem. 1970 Jul;351(7):818–826. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Frehner M., Conn E. E. The Linamarin beta-Glucosidase in Costa Rican Wild Lima Beans (Phaseolus lunatus L.) Is Apoplastic. Plant Physiol. 1987 Aug;84(4):1296–1300. doi: 10.1104/pp.84.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner E., Kiel U. Eine neue Mandelsäurenitril-Lyase (D-Oxynitrilase) aus Prunus laurocerasus (Kirschlorbeer) Hoppe Seylers Z Physiol Chem. 1975 Dec;356(12):1853–1857. [PubMed] [Google Scholar]
- Gerstner E., Pfeil E. Zur Kenntnis des Flavinenzyms Hydroxynitril-Lyase (D-Oxynitrilase. Hoppe Seylers Z Physiol Chem. 1972 Mar;353(3):271–286. doi: 10.1515/bchm2.1972.353.1.271. [DOI] [PubMed] [Google Scholar]
- Ghosh S. A new staining method for nucleoli. Naturwissenschaften. 1974 Dec;61(12):687–688. doi: 10.1007/BF00606526. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Kende H. Enzymes of ethylene biosynthesis. Plant Physiol. 1989 Sep;91(1):1–4. doi: 10.1104/pp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Iwatsuki N., Data E. S., Villegas C. D., Uritani I. Changes of cyanide content and linamarase activity in wounded cassava roots. Plant Physiol. 1983 May;72(1):186–189. doi: 10.1104/pp.72.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Poulton J. E., Thayer S. S., Conn E. E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979 Jun;63(6):1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki G. W., Poulton J. E. Comparison of kinetic and molecular properties of two forms of amygdalin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1986 Jun;247(2):433–439. doi: 10.1016/0003-9861(86)90603-x. [DOI] [PubMed] [Google Scholar]
- Kuroki G. W., Poulton J. E. Isolation and characterization of multiple forms of prunasin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1987 May 15;255(1):19–26. doi: 10.1016/0003-9861(87)90290-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M. J., Johnson K. D., Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989 Jul;90(3):1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkpong O. E., Yan H., Chism G., Sayre R. T. Purification, characterization, and localization of linamarase in cassava. Plant Physiol. 1990 May;93(1):176–181. doi: 10.1104/pp.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Presence of the cyanogenic glucoside dhurrin in isolated vacuoles from sorghum. Plant Physiol. 1978 Feb;61(2):154–157. doi: 10.1104/pp.61.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. S., Conn E. E. Subcellular Localization of Dhurrin beta-Glucosidase and Hydroxynitrile Lyase in the Mesophyll Cells of Sorghum Leaf Blades. Plant Physiol. 1981 Apr;67(4):617–622. doi: 10.1104/pp.67.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yemm R. S., Poulton J. E. Isolation and characterization of multiple forms of mandelonitrile lyase from mature black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1986 Jun;247(2):440–445. doi: 10.1016/0003-9861(86)90604-1. [DOI] [PubMed] [Google Scholar]