Abstract
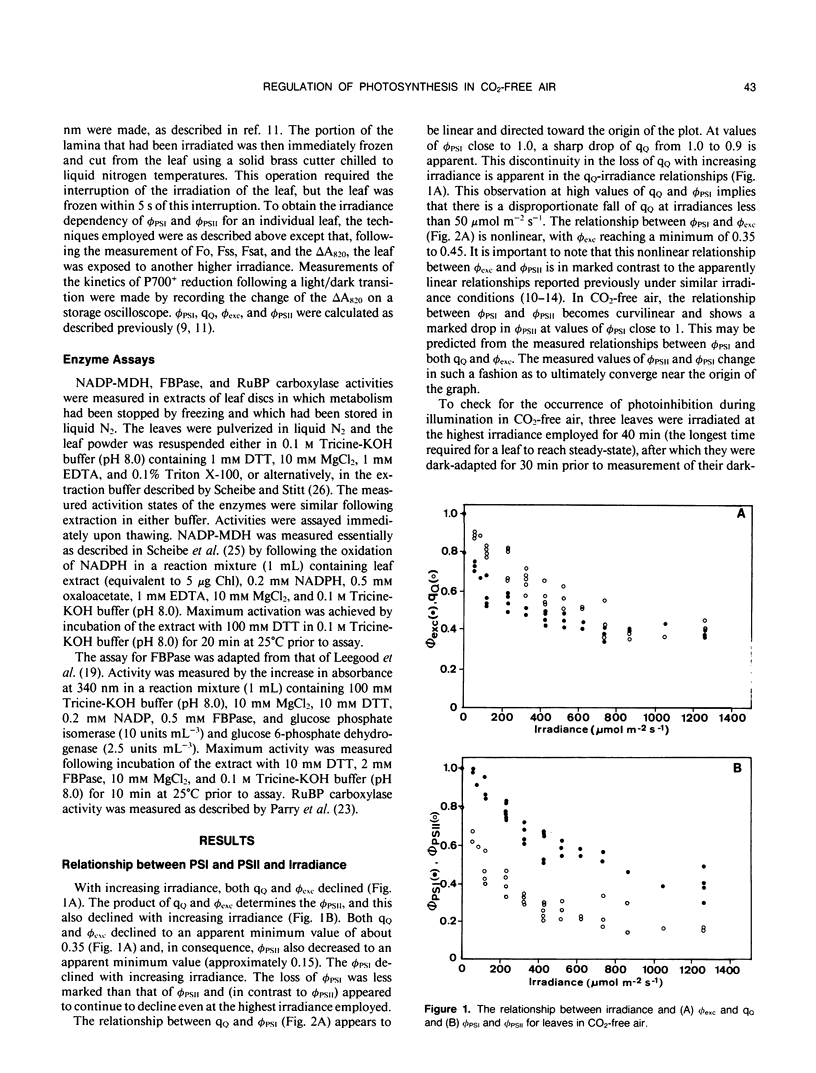
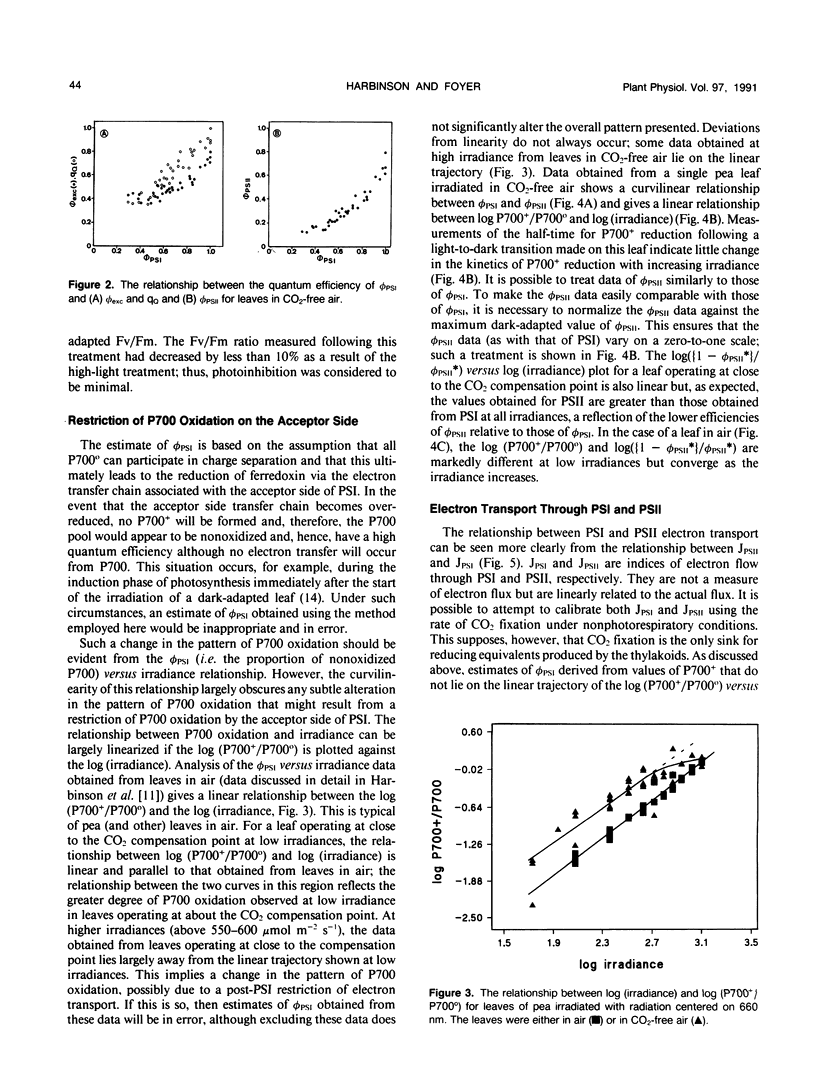
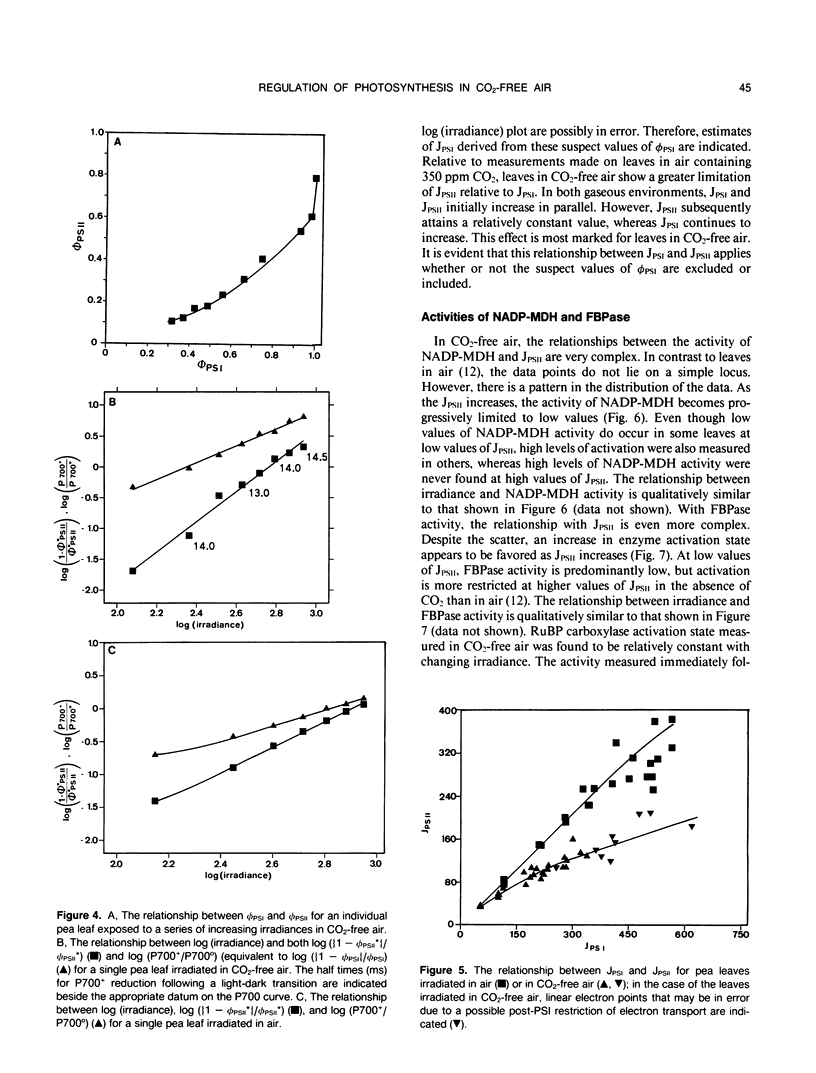
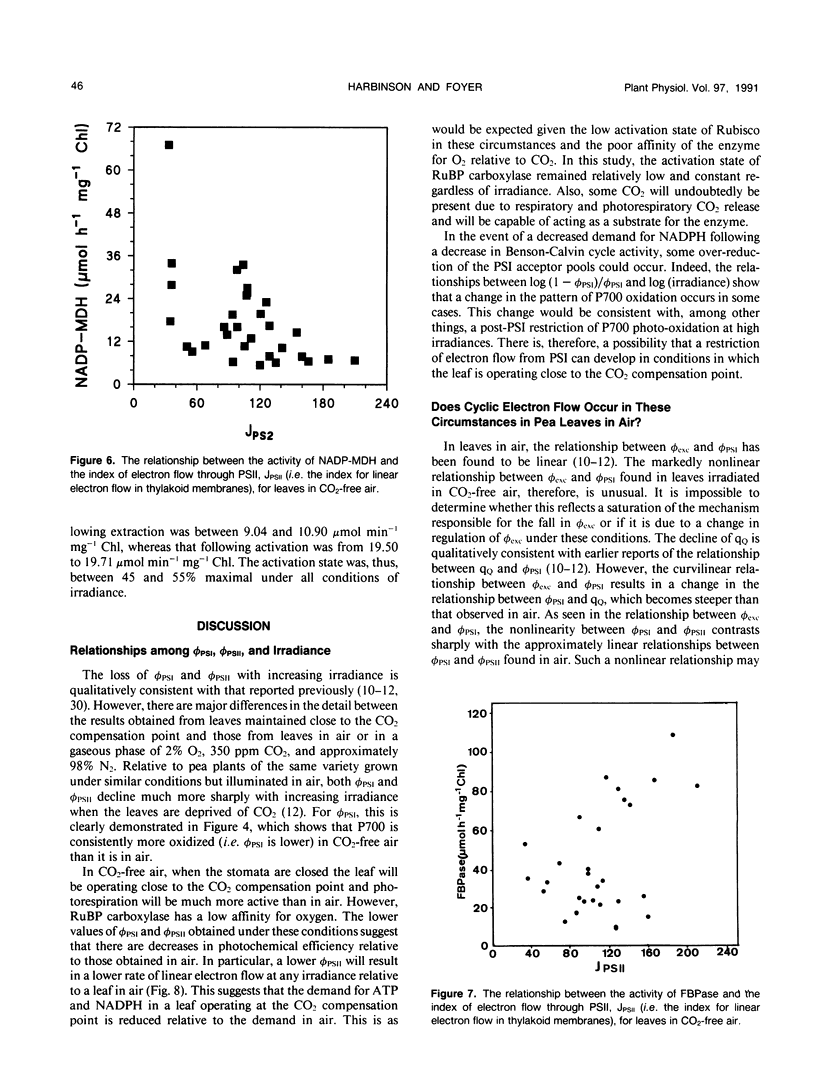
The responses of the efficiencies of photosystems I and II, stromal redox state (as indicated by NADP-malate dehydrogenase activation state), and activation of the Benson-Calvin cycle enzymes ribulose 1,5-bisphosphate carboxylase and fructose 1,6-bisphosphatase to varying irradiance were measured in pea (Pisum sativum L.) leaves operating close to the CO2 compensation point. A comparison of the relationships among these parameters obtained from leaves in air was made with those obtained when the leaves were maintained in air from which the CO2 had been removed. P700 was more oxidized at any measured irradiance in CO2-free air than in air. The relationship between the quantum efficiencies of the photosystems in CO2-free air was distinctly curvilinear in contrast to the predominantly linear relationship obtained with leaves in air. This nonlinearity may be consistent with the operation of cyclic electron flow around photosystem I because the quantum efficiency of photosystem II was much more restricted than the quantum efficiency of photosystem I. In CO2-free air, measured NADP-malate dehydrogenase activities varied considerably at low irradiances. However, at high irradiance the activity of the enzyme was low, implying that the stroma was oxidized. In contrast, fructose-1,6-bisphosphatase activities tended to increase with increasing electron flux through the photosystems. Ribulose-1,5-bisphosphate carboxylase activity remained relatively constant with respect to irradiance in CO2-free air, with an activation state 50% of maximum. We conclude that, at the CO2 compensation point and high irradiance, low redox states are favored and that cyclic electron flow may be substantial. These two features may be the requirements necessary to trigger and maintain the dissipative processes in the thylakoid membrane.
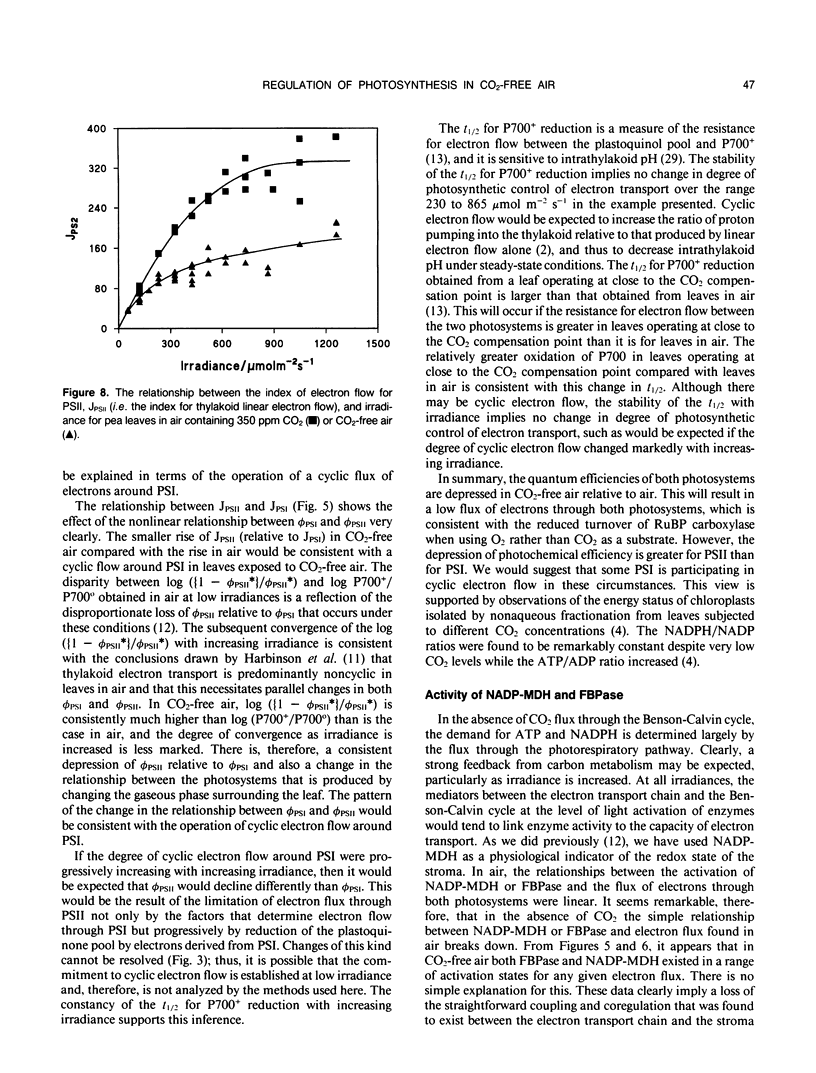
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford N. A., Droux M., Kosower N. S., Buchanan B. B. Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch Biochem Biophys. 1989 May 15;271(1):223–239. doi: 10.1016/0003-9861(89)90273-7. [DOI] [PubMed] [Google Scholar]
- Dujardyn M., Foyer C. H. Limitation of CO(2) Assimilation and Regulation of Benson-Calvin Cycle Activity in Barley Leaves in Response to Changes in Irradiance, Photoinhibition, and Recovery. Plant Physiol. 1989 Dec;91(4):1562–1568. doi: 10.1104/pp.91.4.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbinson J., Genty B., Baker N. R. Relationship between the Quantum Efficiencies of Photosystems I and II in Pea Leaves. Plant Physiol. 1989 Jul;90(3):1029–1034. doi: 10.1104/pp.90.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Kaiser W. M., Förster J. Low CO(2) Prevents Nitrate Reduction in Leaves. Plant Physiol. 1989 Nov;91(3):970–974. doi: 10.1104/pp.91.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace G. M., Volk R. J., Jackson W. A. Nitrate Reduction in Response to CO(2)-Limited Photosynthesis : Relationship to Carbohydrate Supply and Nitrate Reductase Activity in Maize Seedlings. Plant Physiol. 1990 Feb;92(2):286–292. doi: 10.1104/pp.92.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. A., Keys A. J., Foyer C. H., Furbank R. T., Walker D. A. Regulation of ribulose-1,5-bisphosphate carboxylase activity by the activase system in lysed spinach chloroplasts. Plant Physiol. 1988 Jul;87(3):558–561. doi: 10.1104/pp.87.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulié J. M., Buc J., Meunier J. C., Pradel J., Ricard J. Molecular properties of chloroplastic thioredoxin f and the photoregulation of the activity of fructose 1,6-bisphosphatase. Eur J Biochem. 1981 Oct;119(3):497–502. doi: 10.1111/j.1432-1033.1981.tb05635.x. [DOI] [PubMed] [Google Scholar]


