Abstract
Background
Exposure to antibiotics has been shown to be one of the drivers of antimicrobial resistance (AMR) and is critical to address when planning and implementing strategies for combatting AMR. However, data on antibiotic use in sub-Saharan Africa are still limited. Using hospital-based surveillance data from the African Network for Improved Diagnostics, Epidemiology and Management of Common Infectious Agents (ANDEMIA), we assessed self-reported antibiotic use in multiple sub-Saharan African countries.
Methods
ANDEMIA included 12 urban and rural health facilities in Côte d’Ivoire, Burkina Faso, Democratic Republic of the Congo, and Republic of South Africa. Patients with acute respiratory infection (RTI), acute gastrointestinal infection (GI) and acute febrile disease of unknown cause (AFDUC) were routinely enrolled, and clinical, demographic, socio-economic and behavioral data were collected using standardized questionnaires. An analysis of ANDEMIA data from February 2018 to May 2022 was conducted. Reported antibiotic use in the ten days prior to study enrolment were described by substance and by the WHO AWaRe classification (“Access”, “Watch”, “Reserve”, and “Not recommended” antibiotics). Frequency of antibiotic use was stratified by location, disease syndrome and individual patient factors.
Results
Among 19,700 ANDEMIA patients, 7,258 (36.8%) reported antibiotic use. A total of 9,695 antibiotics were reported, including 54.7% (n = 5,299) from the WHO Access antibiotic group and 44.7% (n = 4,330) from the WHO Watch antibiotic group. The Watch antibiotic ceftriaxone was the most commonly reported antibiotic (n = 3,071, 31.7%). Watch antibiotic use ranged from 17.4% (56/322) among RTI patients in Côte d’Ivoire urban facilities to 73.7% (630/855) among AFDUC patients in Burkina Faso urban facilities. Reported antibiotic use included WHO Not recommended antibiotics but no Reserve antibiotics.
Conclusions
Reported antibiotic use data from this multicenter study in sub-Saharan Africa revealed a high proportion of WHO Watch antibiotics. Differences in Watch antibiotic use were found by disease syndrome, country and health facility location, which calls for a more differentiated approach to antibiotic use interventions including further evaluation of accessibility and affordability of patient treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-024-01365-w.
Keywords: Antimicrobial resistance, Antibiotic use, WHO AWaRe classification, Low- and middle-income countries, Sub-saharan Africa
Background
Antimicrobial resistance (AMR) poses a major global health threat. It was estimated that bacterial AMR was associated with 4.95 million deaths worldwide in 2019 [1]. It was highest in western sub-Saharan Africa, the estimated burden of people dying due to infections with resistant pathogens was 23.7 per 100,000 (95% Confidence Interval [CI]: 18.2–30.7 per 100,000), which is 7.3 per 100,000 above the global mean [1].
Exposure to antibiotics has been shown to be a driver of AMR in certain settings [2] and is therefore, critical to address when planning and implementing strategies for combatting AMR [1, 3, 4].
A systematic assessment of antibiotic consumption per capita across 76 countries from 2000 to 2015 by Klein et al. revealed an alarming overall increase of 39% in the antibiotic consumption rate over the study period (11.3 to 15.7 DDDs per 1,000 inhabitants per day) [5]. The total per capita consumption of antibiotics was considerably lower in low- and middle-income countries (LMICs) compared to high-income countries (HICs), although there was a large relative increase in antibiotic consumption over the given period in LMICs [5], highlighting the importance of intensified research on antibiotic use across different settings.
To monitor antibiotic use and guide the implementation of antimicrobial stewardship policies, the World Health Organization (WHO) introduced the AWaRe classification framework in 2017, which groups antibiotics into four categories: “Access” (i.e. essential first- or second-line empiric treatment), “Watch” (i.e. antibiotics with a higher risk for selection of resistance and for specific indications only), “Reserve” (i.e. last resort antibiotics for confirmed or highly suspected infections due to multi-drug resistant organisms) and “Not Recommended” antibiotics (i.e. antibiotics or combinations that are not recommended) [6, 7]. In the “Adopt AWaRe” campaign, WHO proposed a target that by 2023 at least 60% of national antibiotic consumption should come from the Access antibiotics group [8].
Another study by Klein et al., applying the AWaRe classification, demonstrated global per capita increases in the consumption of Watch group antibiotics by 90.9% and Access group antibiotics by 26.2% from 2000 to 2015 [9]. The increase in Watch antibiotic consumption was greater in LMICs (165%) compared to HICs (27.9%). Furthermore, the proportion of countries where Access antibiotics accounted for at least 60% of the total antibiotic consumption decreased from 76% (50/66) of countries in 2000 to 55% (42/76) of countries in 2015. In the first study, Klein et al. had found that the consumption rate of cephalosporins, macrolides and quinolones increased among LMICs. The consumption of broad-spectrum penicillins, carbapenems and polymyxins had increased across all country income groups, with a pronounced increase in upper middle-income countries [5].
However, in these global assessments of antibiotic consumption, LMICs, particularly those in sub-Saharan Africa, were largely excluded due to lack of data. In many LMICs, there is a high burden of infectious diseases and infections caused by antimicrobial resistant organisms [1, 10]. Yet, access to diagnostic testing and affordable antibiotic treatment options often remains limited in these settings [11–14]. Other health system factors such as lack of health care infrastructure, available personnel or substandard medications may also further complicate the situation of antimicrobial use across low-resource settings. These factors may lead to empiric or self-medication with inappropriate or inadequate antibiotics and/or incorrect dosing, although a holistic approach is needed when considering the complex range of factors affecting antimicrobial use in such settings [15–21].
Generating antibiotic consumption or use data is, thus, of utmost importance to evaluate antimicrobial stewardship efforts especially in low-resource settings. To address this gap, we aimed to describe reported initial antibiotic use among patients with respiratory infection (RTI), gastrointestinal infection (GI) and acute fever of unknown origin (AFDUC) in a multicenter hospital-based surveillance study in sub-Sahara Africa. We sought to assess self-reported antibiotic use according to the WHO AWaRe classification and by location and patient factors in order to provide further insights into antibiotic use in these settings where such data are urgently needed.
Methods
Study design and population
A descriptive analysis of data from the ANDEMIA (African Network for Improved Diagnostics, Epidemiology and Management of Common Infectious Agents) infectious disease surveillance network was conducted using data from 1st February 2018 to 26th May 2022. ANDEMIA is a transnational sentinel surveillance network including 12 urban and rural sentinel health care facilities in Côte d’Ivoire (CIV), Burkina Faso (BF), Democratic Republic of Congo (DRC) and Republic of South Africa (RSA). Country and study site characteristics are provided in the supplementary text (see Additional File 1 and 2) [22].
Data collection
Since 2018, the ANDEMIA surveillance network has been collecting clinical, epidemiological, and laboratory data on patients with acute febrile disease of unknown cause (AFDUC), community-acquired gastrointestinal infection (GI), and respiratory tract infection (RTI). The network aims to expand the understanding of etiologies causing these syndromes, including AMR, and to build capacity for infection prevention and control [22]. Data collection procedures including case definitions were previously described by Schubert et al. (see Additional File 1 and 2) [22]. In summary, efforts were made to enroll patients in the 24 h following presentation to the health care facility. Following informed consent and study enrolment, trained staff completed the case investigation form together with the participants or caregivers for minors prior to collection of study specimens. This questionnaire comprised questions on patient demographics, current symptoms, medical history, past and current hospitalization, medication as well as data on individual occupation, socio-economics, housing, water, sanitation, and hygiene (WaSH) facilities, and animal exposures. Antibiotic treatment, including name and date of last dose, taken in the last ten days prior to study inclusion was recorded (see antibiotic use questions in Case Investigation Form in Additional file 3) and therefore subsumes (1) patient self-medication with antibiotics before presentation to the health facility in the last ten days prior to enrolment, (2) prescribed antibiotic use before presentation to the health facility in the last ten days prior to enrolment and, (3) antibiotic use in the enrolling health facility before enrolment if the study samples had not yet been collected. All data were entered into an ANDEMIA study customized database by trained data clerks. Regular plausibility checks, data management reports and validations as well as regular trainings were carried out to improve data quality as described in the published ANDEMIA study protocol [22].
Data analysis
For this study, selected questionnaire data were extracted and further cleaned. All analyses were performed using Stata (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). Antibiotic use was defined as self-reported use of one or more antibiotics in the ten days prior to study enrolment, including those with a valid date of the last dose recorded on the case investigation form. Antibiotics were analysed by total reported antibiotics and by patients who received one or more antibiotics. Antibiotics that were erroneously reported elsewhere on the case investigation form (e.g. under “other medication”) and antibiotics for which no date was given were summarised separately. Reported antibiotics were grouped according to the WHO AWaRe classification: “Access”, “Watch”, “Reserve”, and “Not Recommended” antibiotics [6]. Guided by the essential medicine lists of the ANDEMIA countries [23–26], the route of administration of reported antibiotics was coded according to expert clinical opinion into the following strata: “parenteral only” (i.e. only parenteral formulation exists), “oral only”, and “both (parenteral and oral) or other possible routes of administration”. If the essential medicine lists in the countries differed, the reported antibiotic was coded in the third strata “both (parenteral and oral) or other possible routes of administration”. The coding frameworks for the antibiotic formulation and WHO AWaRe criteria are provided in the supplementary text (see Additional File 4). Other variables such as climatic region, dry/wet zone, enrolment before or during the COVID-19 pandemic were also created. The start date of the pandemic was set to 11 March 2020 as it was officially declared by the WHO. Biometric measures were calculated according to WHO recommendations including the Z-score with percentiles for 0–5 years [27] and 5–19 years [28] and the Body Mass Index (BMI) classification for adults [29]. These biometric measurement results were combined into a single variable for weight classification ranging from underweight, normal weight, overweight to obesity (see Additional file 5).
We first described the overall ANDEMIA study population by country and patient factors. The absolute and relative frequencies of antibiotic use among enrolled patients were then analysed by country, urban or rural health care facility, patient infectious syndrome, age group, sex, level of education, employment (if minor, of the respective parent or legal caregiver), residence (reported village or city), the time of study enrolment (before or during the COVID-19 pandemic), and clinical factors, weight classification, reported co-morbidities, other medications (antimalarials, antiretrovirals, anti-tuberculosis agents as well as other medicines), the onset of symptoms and current hospitalization. The reported antibiotics were classified by antibiotic agent and according to the WHO AWaRe classification and analysed by health facility location, syndrome complex and patient factors. The ratio of Access-to-Watch antibiotics was calculated in the different patient groups. The number of antibiotics per patient and routes of antibiotic administration were also summarised overall and by health facility location and patient factors. A sensitivity analysis restricting antibiotic use reported only on the same day of enrolment (versus in the full period of ten days prior to study enrolment) was also conducted.
Ethics
The ANDEMIA network surveillance study adheres to the respective national legislation and ethical standards as well as the Declaration of Helsinki. In all participating countries including Germany, institutional ethics approval was obtained (see the Declaration section below for details). ANDEMIA study objectives were explained, either verbally or in writing, to all study participants (in the case of minors, to parents or legal caregivers) by trained surveillance officers and, prior to obtaining written informed consent. Patient data were pseudonymized upon entry into the database and only accessible to selected trained study staff [22].
Results
Overall characteristics of ANDEMIA study participants
Between 1 February 2018, and 26 May 2022, 19,700 patients were enrolled in the ANDEMIA study, including 5,529 (28.1%) from CIV; 4,802 (24.4%) from BF; 5,937 (30.1%) from DRC and 3,432 (17.4%) from RSA. Across all countries, 36.6% (n = 7,203) of patients presented with AFDUC, 33.9% (n = 6,676) with RTI, 25.8% (n = 5,085) with GI and 3.7% (n = 736) with both RTI and GI. Approximately half of enrolled patients were children under the age of five and 48.6% were female. More were recruited in health facilities from urban areas (n = 12,527, 63.6%) than from rural areas (n = 7,173, 36.4%), although 44.0% (8,624/19,617) of overall enrolled patients with data on place of residence reported living in a village (this included 2,413 patients recruited from urban health facilities). A greater proportion of adult patients or legal guardians of minors reported no education in CIV (n = 2,987, 54.0%) and BF (n = 3,166, 66.4%) compared to DRC (n = 876, 14.8%) and RSA (n = 63, 1.9%). During the study period, approximately half of patients were enrolled before the COVID-19 pandemic (n = 10,604, 53.8%). Although data showed a hospitalization rate of 98.6% (11,009/11,186), data on hospitalization were largely incomplete for CIV (51.8%; 2,861 missing entries) DRC (47.3%; 2,809 missing entries) and BF (29.3%, 1,408 missing entries). A summary table of patient characteristics by country is provided in the supplementary text (see Additional File 6).
Characteristics of study participants reporting antibiotic use
Among 19,700 ANDEMIA patients, 7,258 (36.8%) reported antibiotic use in the ten days prior to study enrolment. During the study period, slightly more than half of patients with self-reported antibiotic use were enrolled before the COVID-19 pandemic (n = 4,319, 59.5%). In CIV, BF, and DRC, more than 80% of patients with reported antibiotic use presented to urban health facilities, in contrast to RSA where the presentation to urban and rural health facilities was fairly balanced including 51.8% presenting to rural and 48.2% to urban facilities. About half of the patients with reported antibiotic use from BF (49.9%) and from RSA (52.1%) reported living in a village in contrast to 37.5% in CIV and 27.7% in DRC. Slightly more than half of enrolled patients with reported antibiotic use were children under the age of five (n = 4,077, 57.0%) and 45.9% were female. A greater proportion of adult patients or legal guardians of minors reported no education in CIV (n = 354, 53.7%) and BF (n = 1,388, 56.6%) compared to DRC (n = 315, 16.6%) and RSA (n = 42, 1.9%). Approximately half of the patients or legal guardians of minors who reported antibiotic use were unemployed, ranging from 21.5% (n = 495) in BF to 72.6% (n = 1,592) in RSA. Among those with data on hospitalization, 99.3% of the patients with reported antibiotic use were currently hospitalized at enrolment, although data on hospitalization was incomplete, particularly in CIV (45.4%, 299/659 missing entries) and DRC (19.9%, 378/1,903 missing entries) (see Table 1).
Table 1.
Characteristics of study participants reporting antibiotic use ten days prior to study enrolment by country
| Country | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 7,258) |
Côte d’Ivoire (N = 659) |
Burkina Faso (N = 2,470) |
DR Congo (N = 1,903) |
Rep. South Africa (N = 2,226) |
||||||
| n | % | n | % | n | % | n | % | n | % | |
| Syndrome | ||||||||||
| AFDUC | 2,198 | 30.3% | 187 | 28.4% | 879 | 35.6% | 514 | 27.0% | 618 | 27.8% |
| GI | 1,642 | 22.6% | 207 | 31.4% | 355 | 14.4% | 538 | 28.3% | 542 | 24.3% |
| RTI | 3,009 | 41.5% | 261 | 39.6% | 1,161 | 47.0% | 665 | 34.9% | 922 | 41.4% |
| GI/RTI | 409 | 5.6% | 4 | 0.6% | 75 | 3.0% | 186 | 9.8% | 144 | 6.5% |
| Covid-19 pandemic | ||||||||||
| Enrolled before | 4,319 | 59.5% | 508 | 77.1% | 1,309 | 53.0% | 1,181 | 62.1% | 1,321 | 59.3% |
| Enrolled during | 2,939 | 40.5% | 151 | 22.9% | 1,161 | 47.0% | 722 | 37.9% | 905 | 40.7% |
| Health facility | ||||||||||
| Rural site | 1,974 | 27.2% | 86 | 13.1% | 357 | 14.5% | 379 | 19.9% | 1,152 | 51.8% |
| Urban site | 5,284 | 72.8% | 573 | 86.9% | 2,113 | 85.5% | 1,524 | 80.1% | 1,074 | 48.2% |
| Patient’s residence* | N = 7,212 | N = 659 | N = 2,451 | N = 1,902 | N = 2,200 | |||||
| Village | 3,142 | 43.6% | 247 | 37.5% | 1,222 | 49.9% | 526 | 27.7% | 1,147 | 52.1% |
| City/Town | 4,070 | 56.4% | 412 | 62.5% | 1,229 | 50.1% | 1,376 | 72.3% | 1,053 | 47.9% |
| Age group* | N = 7,155 | N = 659 | N = 2,465 | N = 1,899 | N = 2,132 | |||||
| <1 year | 1,947 | 27.2% | 101 | 15.3% | 463 | 18.8% | 721 | 38.0% | 662 | 31.1% |
| 1–4 years | 2,130 | 29.8% | 170 | 25.8% | 634 | 25.7% | 675 | 35.5% | 651 | 30.5% |
| 5–17 years | 635 | 8.9% | 61 | 9.3% | 231 | 9.4% | 206 | 10.8% | 137 | 6.4% |
| 18–44 years | 1,269 | 17.7% | 167 | 25.3% | 535 | 21.7% | 163 | 8.6% | 404 | 18.9% |
| ≥45 years | 1,174 | 16.4% | 160 | 24.3% | 602 | 24.4% | 134 | 7.1% | 278 | 13.0% |
| Sex* | N = 7,243 | N = 659 | N = 2,463 | N = 1,903 | N = 2,218 | |||||
| Male | 3,916 | 54.1% | 344 | 52.2% | 1,458 | 59.2% | 1,015 | 53.3% | 1,099 | 49.5% |
| Female | 3,327 | 45.9% | 315 | 47.8% | 1,005 | 40.8% | 888 | 46.7% | 1,119 | 50.5% |
| Level of education* | N = 7,215 | N = 659 | N = 2,452 | N = 1,900 | N = 2,204 | |||||
| No level of education | 2,099 | 29.1% | 354 | 53.7% | 1,388 | 56.6% | 315 | 16.6% | 42 | 1.9% |
| ≤ 6 years | 1,459 | 20.2% | 114 | 17.3% | 578 | 23.6% | 576 | 30.3% | 191 | 8.7% |
| 7–10 years | 1,712 | 23.7% | 85 | 12.9% | 334 | 13.6% | 678 | 35.7% | 615 | 27.9% |
| > 10 years | 1,945 | 27.0% | 106 | 16.1% | 152 | 6.2% | 331 | 17.4% | 1,356 | 61.5% |
| Employment* | N = 7,042 | N = 649 | N = 2,304 | N = 1,895 | N = 2,194 | |||||
| Unemployed | 3,497 | 49.7% | 271 | 41.8% | 495 | 21.5% | 1,139 | 60.1% | 1,592 | 72.6% |
| Self-employed | 2,140 | 30.4% | 267 | 41.1% | 1,327 | 57.6% | 490 | 25.9% | 56 | 2.6% |
| Part time employed | 391 | 5.6% | 16 | 2.5% | 152 | 6.6% | 113 | 6.0% | 110 | 5.0% |
| Full time employed | 1,014 | 14.4% | 95 | 14.6% | 330 | 14.3% | 153 | 8.1% | 436 | 19.9% |
| Hospitalized at enrolment¥ | N = 6,447 | N = 360 | N = 2,344 | N = 1,525 | N = 2,218 | |||||
| No | 42 | 0.7% | 3 | 0.8% | 28 | 1.2% | 10 | 0.7% | 1 | 0.1% |
| Yes | 6,405 | 99.3% | 357 | 99.2% | 2,316 | 98.8% | 1,515 | 99.3% | 2,217 | 99.9% |
Legend: Enrolment period 1 February 2018 till 26 May 2022; AFDUC: acute febrile disease of unknown cause; GI: gastrointestinal infection; RTI: respiratory tract infection; DR Congo: Democratic Republic of the Congo; Rep. South Africa: Democratic Republic of South Africa; *Variables with missing data (< 5%); ¥Missing data exceeds 5%
Differences in characteristics of patients who did and did not report antibiotic use across all patients enrolled in the study period can be seen in Additional file 7.
Among the 7,258 patients who reported antibiotic use in the ten days prior to study enrolment, 3,009 (41.5%) were enrolled with RTI, 2,198 (30.3%) with AFDUC, and 1,642 (22.6%) with GI (Tables 1 and 2). Over 90% (n = 6,957) of patients who reported antibiotic use had taken the last dose of the reported antibiotic within 2 days prior to study enrolment. Across all syndromes, 45.7% (3,276/7,170) reported symptom onset in the 4–7 days prior to enrolment. Among those with the GI syndrome, 48.2% (757/1,569) reported onset of symptoms in the 0–3 days prior to enrolment.
Table 2.
Characteristics of study participants reporting antibiotic use in the ten days prior to enrolment by syndrome
| Syndrome enrolment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total1 | AFDUC | GI | RTI | GI/RTI | |||||||
| N = 7,258 | N = 2,198 | N = 1,642 | N = 3,009 | N = 409 | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Last dose of reported antibiotic° | |||||||||||
| Same day | 3,860 | 53.2% | 1,188 | 54.0% | 880 | 53.6% | 1,619 | 53.8% | 173 | 42.3% | |
| 1–2 days ago | 3,097 | 42.7% | 916 | 41.7% | 697 | 42.4% | 1,272 | 42.3% | 212 | 51.8% | |
| 3–10 days ago | 301 | 4.1% | 94 | 4.3% | 65 | 4.0% | 118 | 3.9% | 24 | 5.9% | |
| Symptom onset* | N = 7,170 | N = 2,191 | N = 1,569 | N = 3,007 | N = 403 | ||||||
| 0–3 days ago | 2,763 | 38.5% | 881 | 40.2% | 757 | 48.2% | 996 | 33.1% | 129 | 32.0% | |
| 4–7 days ago | 3,276 | 45.7% | 942 | 43.0% | 681 | 43.4% | 1,421 | 47.3% | 232 | 57.6% | |
| 8–10 days ago | 1,131 | 15.8% | 368 | 16.8% | 131 | 8.3% | 590 | 19.6% | 42 | 10.4% | |
| Sex and age* | N = 7,140 | N = 2,142 | N = 1,624 | N = 2,970 | N = 404 | ||||||
| Male, < 5 years | 2,286 | 32.0% | 524 | 24.5% | 673 | 41.4% | 882 | 29.7% | 207 | 51.2% | |
| Female, < 5 years | 1,784 | 25.0% | 407 | 19.0% | 495 | 30.5% | 733 | 24.7% | 149 | 36.9% | |
| Male, ≥ 5 years | 1,578 | 22.1% | 642 | 30.0% | 192 | 11.8% | 728 | 24.5% | 16 | 4.0% | |
| Female, ≥ 5 years | 1,492 | 20.9% | 569 | 26.6% | 264 | 16.3% | 627 | 21.1% | 32 | 7.9% | |
| Weight category¥ | N = 5,325 | N = 1,600 | N = 1,100 | N = 2,364 | N = 261 | ||||||
| Underweight | 1,749 | 32.8% | 401 | 25.1% | 514 | 46.7% | 698 | 29.5% | 136 | 52.1% | |
| Normal | 2,676 | 50.3% | 919 | 57.4% | 420 | 38.2% | 1,240 | 52.5% | 97 | 37.2% | |
| Overweight | 569 | 10.7% | 185 | 11.6% | 99 | 9.0% | 264 | 11.2% | 21 | 8.0% | |
| Obese | 331 | 6.2% | 95 | 5.9% | 67 | 6.1% | 162 | 6.9% | 7 | 2.7% | |
| Co-morbidities* | N = 7,181 | N = 2,184 | N = 1,627 | N = 2,964 | N = 406 | ||||||
| No | 6,612 | 92.1% | 2,017 | 92.4% | 1,546 | 95.0% | 2,676 | 90.3% | 373 | 91.9% | |
| Yes | 569 | 7.9% | 167 | 7.6% | 81 | 5.0% | 288 | 9.7% | 33 | 8.1% | |
| Antimalarials* | N = 7,036 | N = 2,154 | N = 1,600 | N = 2,884 | N = 398 | ||||||
| No | 5,631 | 80.0% | 1,684 | 78.2% | 1,233 | 77.1% | 2,423 | 84.0% | 291 | 73.1% | |
| Yes | 1,405 | 20.0% | 470 | 21.8% | 367 | 22.9% | 461 | 16.0% | 107 | 26.9% | |
| Other medication* | N = 6,992 | N = 2,144 | N = 1,581 | N = 2,872 | N = 395 | ||||||
| No | 3,391 | 48.5% | 1,029 | 48.0% | 713 | 45.1% | 1,506 | 52.4% | 143 | 36.2% | |
| Yes | 3,601 | 51.5% | 1,115 | 52.0% | 868 | 54.9% | 1,366 | 47.6% | 252 | 63.8% | |
Legend 1: Enrolment from 1 February 2018 till 26 May 2022. AFDUC: acute febrile disease of unknown cause; GI: gastrointestinal infection; RTI: respiratory tract infection. ° only first reported antibiotic shown *Variables with missing data < 5%. ¥Missing data exceeds 5%. 1 For a full row frequency table indicating characteristics of study participants who reported antibiotic use by syndrome see Additional file 8
Half of the patients (2,676/5,325, 50.5%) with available BMI/z-score data who reported antibiotic use were of normal weight, 32.8% (1,749/5,325) were underweight, and 16.9% (900/5,325) were overweight or obese. In particular, approximately half of patients with a GI or GI/RTI syndrome who reported antibiotic use were underweight (514/1,100, 46.7%; and 136/261, 52.1% respectively). Among those with treatment data, 20.0% (1,405/7,036) of patients who reported antibiotic use also reported use of antimalarials and 51.5% (3,601/6,992) reported use of other medication such as analgesics, dietary supplements, antiparasitic drugs (other than antimalarials) and corticosteroids, with only minor differences between the syndrome enrolment (Table 2).
Most commonly reported antibiotics
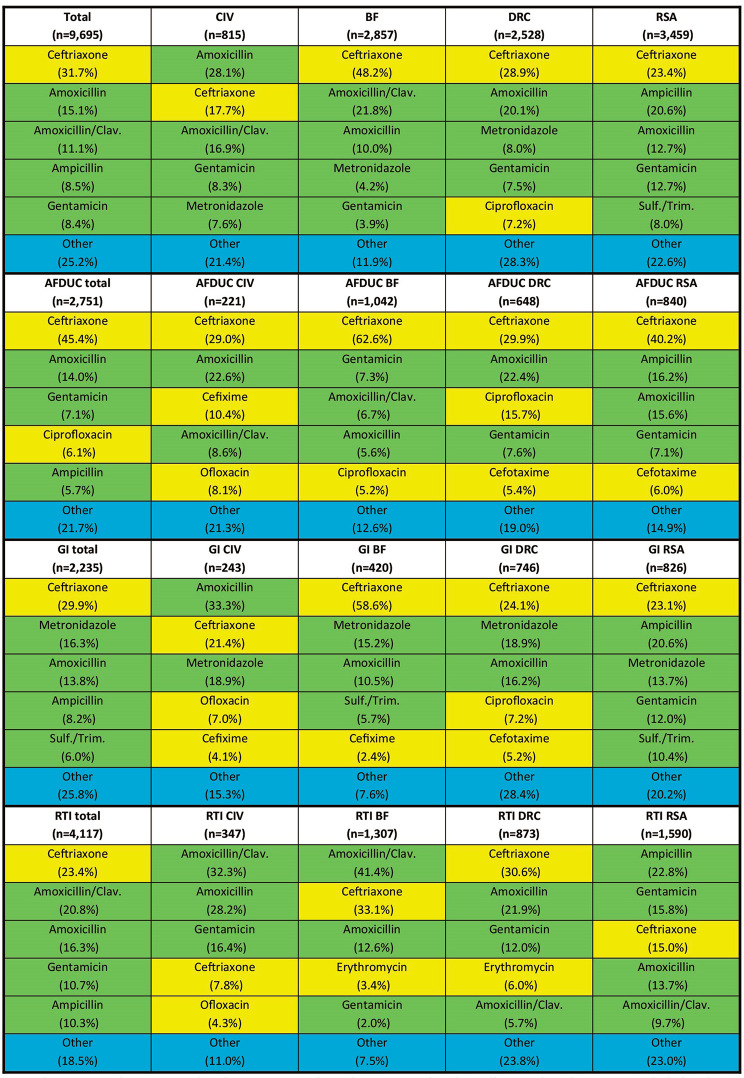
Among 7,258 patients who reported antibiotic use, 9,695 antibiotics were reported in the ten days prior to study enrolment. The most common antibiotic reported was ceftriaxone (31.7%, n = 3,071), a WHO Watch antibiotic, followed by amoxicillin (15.1%, n = 1,466) and amoxicillin/clavulanic acid (11.1%, n = 1,075), both WHO Access antibiotics. Among patients with the AFDUC syndrome, ceftriaxone was the most commonly reported antibiotic, ranging from 29.0% in CIV (64/221) to 62.6% (652/1,042) in BF. Among patients with GI, ceftriaxone (ranging from 21.4% (52/243) in CIV to 58.6% (246/420) in BF), and metronidazole (ranging from 13.7% (113/826) in RSA to 18.9% in DRC and CIV (141/746 and 46/243 respectively) were the most commonly reported antibiotics. Among those with RTI, amoxicillin/clavulanic acid was the most commonly reported antibiotic in CIV (32.3%; 112/347) and BF (41.4%; 541/1,307). Apart from ceftriaxone, other Watch group antibiotics such as ciprofloxacin (in particular, 6.1% (169/2,751) of patients with AFDUC), cefotaxime, cefixime, ofloxacin and erythromycin were reported (Fig. 1). In total, only three (0.03%) carbapenems (imipenem once, meropenem twice) were reported. Of the 66 reported antibiotics belonging to the Not recommended group, 63 (95.5%) were reported from DRC. A majority of the 63 Not recommended group antibiotics reported in DRC came from the fixed-dose-combination metronidazole/norfloxacin (71.4% or 45/63) and primarily among patients with the GI and GI/RTI syndrome (66.7% or 42/63).
Fig. 1.
Top five most commonly reported antibiotics overall and by country, syndrome and WHO AWaRe classification. Legend: Access antibiotics are colored in green, Watch antibiotics are colored in yellow, and antibiotics not included in the top five reported antibiotics are grouped as “other” and colored in blue. Sulf./Trim. = Sulfamethoxazole/Trimethoprim; Amoxicillin/Clav. = Amoxicillin/clavulanic acid*. AFDUC: acute febrile disease of unknown cause; GI: gastrointestinal infection; RTI: respiratory tract infection; Combination of GI/RTI cases not shown separately (n = 592). CIV: Côte d’Ivoire, BF: Burkina Faso, DRC: The Democratic Republic of the Congo, RSA: The Republic of South Africa
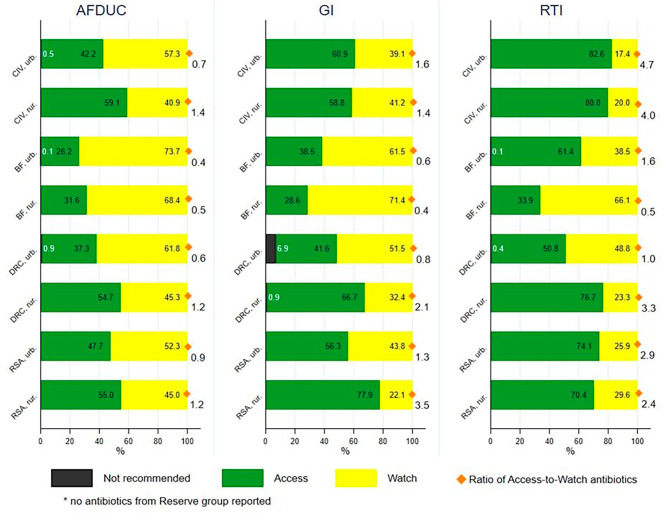
Among the top five antibiotics reported across countries, only Access and Watch antibiotics were found. Out of all 9,695 reported antibiotics, 54.7% (n = 5,299) were Access, 44.7% (n = 4,330) were Watch, 0.7% (n = 66) were Not recommended, and 0% (n = 0) were Reserve group antibiotics. The overall ratio of Access to Watch antibiotics was 1.2. This varied by country, urban and rural health facilities and syndrome, (Fig. 2) ranging from 0.4 among rural facilities in BF to 4.7 among urban facilities in CIV. Among those with the AFDUC syndrome, approximately half or less of reported antibiotics were Access antibiotics. Among those with the GI syndrome, a greater number of patient reported antibiotics were Access antibiotics, especially across the urban health facilities in CIV and rural health care facilities in DRC and RSA which reported greater than 60% of Access group antibiotics. Among those with the RTI syndrome, the greatest number of Access antibiotics were.
Fig. 2.
Proportional antibiotic use according to WHO AWaRe classification by syndrome, country and health facility location. Legend: AFDUC: acute fever of unknown cause; GI: gastrointestinal infection; RTI: respiratory tract infection; CIV: Ivory Coast; BF: Burkina Faso; DRC: The Democratic Republic of the Congo; RSA: Republic of South Africa; urb.: urban health centre; rur.: rural health centre
reported including all health care facilities in CIV and RSA as well as rural facilities in DRC reporting more than 70% Access antibiotics (Fig. 2).
Before the COVID-19 pandemic, 40.7% (2,352/5,783) of all reported antibiotics were from the Watch group. This increased to 50.6% (1,978/3,912) during the pandemic (see Additional File 9 for these results by country). The relative increase in the reported use of Watch antibiotics during the pandemic were most pronounced among those with the AFDUC syndrome, from 54.4% (809/1,487) reported antibiotics in AFDUC before the pandemic to 67.6% (855/1,264) during the pandemic, although results varied by country and location. The increase in the reported use of Watch antibiotics from before to during the pandemic were less pronounced among those with GI and RTI, although differences by country and location were also present, particularly in rural RSA where the reported use of Watch among patients with RTI went from 17.3% (84/485) before the pandemic to 42.3% (200/473) during the pandemic.
Due to a missing or an invalid reported date of the last dose, 611 reported antibiotics were excluded from the main analysis. Among these excluded antibiotics, metronidazole was reported in 47.6% (n = 291). A sensitivity analysis of the most commonly reported antibiotics by country and WHO AWaRe classification including these additional antibiotics (n = 10,306) is reported in the supplementary text (see Additional File 10).
Routes of administration and number of reported antibiotics
Of 9,695 total antibiotics reported, 51.6% (n = 4,998) were classified as parenteral use only. Across patients in all countries and by syndrome, these antibiotics included most commonly ceftriaxone, gentamicin, cefotaxime and ampicillin. In BF, ceftriaxone accounted for 90.2% (1,378/1,527) of reported antibiotics classified as parenteral formulations. Of the parenteral classified antibiotics, the last dose was reported on the same day of study enrollment in 52.3% (n = 2,615) of cases, one day prior in 41.9% (n = 2,095) and 3–10 days prior in 5.8% (n = 288) of cases.
The number of different antibiotic substances reported varied considerably between countries and between urban or rural location of the health facilities. While urban DRC reported 37 different antibiotic substances, of which 12 were classified as parenteral, rural CIV, BF and DRC each reported 14 different antibiotic substances, of which two (rural CIV) and four (rural BF and rural DRC) were classified as parenteral formulations (see Additional File 11).
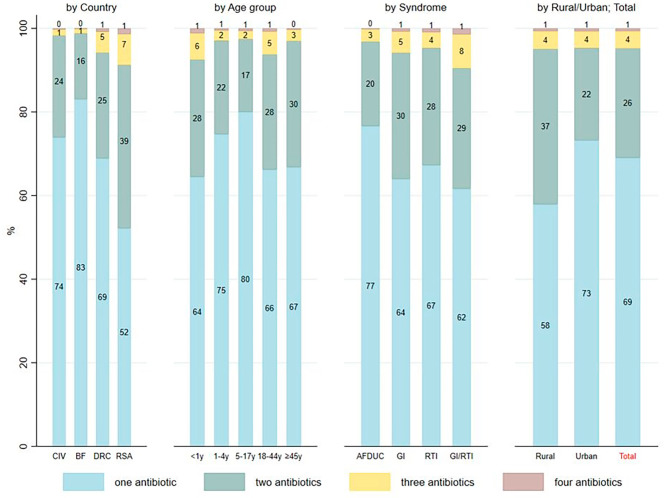
Among the 7,258 patients who received antibiotics, 69.0% (n = 5,010) reported the use of only one antibiotic, followed by 26.2% (n = 1,904) who received two antibiotics, and 4.7% (n = 344) had received three or four antibiotics. In BF, 83.0% (n = 2,051) reported only one antibiotic ten days prior to study enrolment, in contrast to patients in RSA where 52.2% (n = 1,161) reported only one antibiotic. Furthermore, more patients enrolled at urban health care facilities received only one antibiotic (73.2%, 5,284/3,867) before enrolment compared to those at rural health facilities (57.9%, 1,143/1,974) (Fig. 3).
Fig. 3.
Number of antibiotics reported per patient in the ten days prior to study inclusion. Legend: CIV: Côte d’Ivoire; BF: Burkina Faso; DRC: Democratic Republic of the Congo; RSA: Republic of South Africa; AFDUC: acute fever of unknown cause; GI: gastrointestinal infection; RTI: respiratory tract infection
The sensitivity analysis assessing the number of antibiotics reported among patients who reported antibiotic use on the same day as study enrolment only (as opposed to the full time period of ten days prior) showed similar trends.
Discussion
We investigated the self-reported antibiotic use in the ten days prior to enrolment among patients in a large infectious disease surveillance study in sub-Saharan Africa. Use of WHO Watch antibiotics, particularly the third-generation cephalosporin ceftriaxone, was frequently reported. Despite the high burden of infectious diseases due to resistant bacteria in these countries suggested by other studies [1], no antibiotic from the Reserve group was reported. Importantly, Watch antibiotic use varied between the clinical syndromes of AFDUC, GI and RTI as well as by location of participating health facilities including country and urban or rural setting. In three ANDEMIA countries, relative use of Watch antibiotics increased during the COVID-19 pandemic.
A recent review and meta-analysis suggested that the selection of multidrug resistant bacteria is associated more with exposure to antibiotics from the Watch or Reserve groups compared to those from the Access group [30]. High rates of Watch group antibiotics in LMICs, including a large proportion of ceftriaxone, have been described in other antibiotic consumption or use studies [31–35], such as those using antibiotic sales data [5, 9] or point-prevalence surveys [33, 35]. Some of these studies have assessed antibiotic use according to the AWaRe criteria by fever (i.e. main symptom for triggering antibiotic use) [31, 32] and other clinical symptoms.
In this study, we focused on the defined infectious disease syndromes of AFDUC, GI and RTI used in the ANDEMIA surveillance study. These syndrome-specific self-reported antibiotic use findings provide an important contribution to the evidence base given the high burden of these infections in LMICs, where respiratory tract infections (including tuberculosis), enteric infections and other infectious diseases have been found to account for more than 30% of all total causes of deaths in children < 5 years [36, 37].
In our study, although patients with RTI most frequently reported overall antibiotic use (43.1%), the proportion of reported Watch antibiotic use was the lowest among this patient group, and reported antibiotics more frequently included those from the Access group. In contrast, the highest proportion of Watch antibiotic use was among AFDUC patients, including ceftriaxone as the most commonly reported antibiotic among AFUDC and GI patients.
Furthermore, there was a high proportion of reported use of ceftriaxone, a broad-spectrum beta-lactam in our study. It should be acknowledged that ceftriaxone is a relatively easy to administer and well-tolerated parenteral antibiotic. It has been off-patent since 2005 [38] and is generically available in all four ANDEMIA countries.
To guide the empiric use of antibiotics according to the AWaRe criteria, the recently published WHO AWaRe book and the earlier published WHO Essential Medicine List were developed to support treatment decision making [25, 39, 40]. Some of our syndrome-specific findings appeared to be consistent with the recommendations proposed by these two tools. Ceftriaxone is recommended as one of the first-choice antibiotics for severe community acquired pneumonia (CAP), severe enteric fever with risk for fluoroquinolone resistance, and acute bacterial meningitis. In our study, 6.1% of patients with reported antibiotic use presented with meningeal signs. Ceftriaxone is also recommended as a second-choice treatment for acute infectious diarrhea/gastroenteritis and for sepsis in neonates and children [25, 40], which should be considered in our study given that 57.0% of patients with reported antibiotic use were under the age of five.
Among RTI patients in our study, all five reported antibiotics were the first-, or second choice antibiotic for mild, moderate or severe CAP cases for adults or children according to the AWaRe book [40]. Nonetheless, overprescribing or self-medication of antibiotics in (upper) RTI and inappropriate use of ceftriaxone has been previously described also in LMIC settings [34, 41, 42].
Thus, assessments of antibiotic use by individual patient factors, severity of presentation, and if available, further diagnostics remains crucial to assess the appropriateness of antibiotic use in LMICs.
Another finding in the present study is a shift from Access to increased Watch antibiotic use during the COVID-19 pandemic for BF, DRC and RSA. A critical review comparing treatment guidelines for COVID-19 patients across ten different countries in Sub-Saharan Africa found that some guidelines recommended the use of Watch group antibiotics [43], although only one of the ten countries included was an ANDEMIA country (RSA). In addition, a decrease in antibiotic use per capita has been described during the COVID-19 pandemic [44] which is consistent with our findings, although other factors such as COVID-19 associated mitigation measures and lower attendance to hospitals especially in the beginning of the pandemic may also play a role [45]. Thus, confounding factors cannot be excluded and there may be a need for further studies on the influence of the COVID-19 pandemic and antibiotic consumption or use.
In our study, differences in overall reported antibiotic use were seen by country, ranging from 11.9% in CIV to more than half of enrolled patients in RSA and BF reporting antibiotic use. Furthermore, it was found that the overall reported Access antibiotic use in CIV and RSA was more than 60% compared to lower proportions in BF and in DRC. In addition to varying patient clinical presentation, and location of facilities, as well as slightly different enrolment in the countries, other programmatic aspects may also be considered when interpreting these findings. Differences in governance of AMR prevention and control strategies as well as structures for accountability, surveillance and financing across countries may play important roles [14, 46]. Although national action plans (NAPs) on AMR exist in all four ANDEMIA countries [47–49], implementation can pose significant challenges [46, 50, 51]. As demonstrated by this study, the WHO Access, Watch, and Reserve (AWaRe) classification system of antibiotics according to their spectrum of activity and potential to develop resistance offers an objective framework which can be used to guide evaluations of antibiotic use as well as inform the development and implementation of national policies on antimicrobial stewardship. At the health facility level, patients often presented to urban facilities even if their place of residence was in the village. Antibiotic use was more commonly reported from patients presenting at urban facilities, although multiple therapies were more often reported from patients presenting at rural facilities. Rural health facilities may be facing challenges with lack of staff, access to diagnostic tools and drug shortages [52], which may influence self-medication and switch of treatment, leading to several therapies. Also, across the ANDEMIA facilities different antibiotics reported ranged between 14 and 37 antibiotics (including fixed-dose combinations and WHO Not recommended antibiotics). Reasons for the use of Not recommended antibiotics may include limited access to individual antibiotic formulations and lack of enforced regulation [53].
In this study, 0.03% (n = 3) carbapenems (Watch group) and no antibiotics from the Reserve group were reported. This is particularly notable given that the recent global study modelling the burden of AMR estimated that the proportion of third-generation cephalosporin-resistant isolates for Escherichia coli ranged between 20 and 50% in BF, CIV, DRC and RSA, with even higher proportions of 60–80% resistant for Klebsiella pneumoniae and up to 70% carbapenem-resistant isolates for Acinetobacter baumannii (in RSA) [1].
As outlined above, antibiotic availability and accessibility must be considered when interpreting our results. A recent spatial modelling study comparing global antibiotic consumption data and surveys on antibiotic use found large variations of antibiotic usage also within LMICs, suggesting access and availability to be one explanation [54]. Stock-outs and drug shortages are well known in LMICs. A study which collected data on the availability and prices of drugs (including seven antibiotics) in government and church health facilities, private pharmacies as well as informal vendors in DRC and Cameroon found a wide variation in the availability of the antibiotics, ranging from 37% (antibiotic available at 12/34 facilities) to 94% (antibiotic available at 32/34 facilities) in DRC. This study also calculated the median price ratios and daily wages required for a full treatment course with each antibiotic. For DRC, the cost for a full treatment course ranged from 0.55 (doxycycline) to 10.05 (amoxicillin/clavulanic acid) of the equivalent median daily wage [55]. Another study from Ethiopia which calculated the cost of a full treatment course for different infectious diseases found that for treatment of community acquired pneumonia with ceftriaxone (using 1 g i.v. every 12 h for 7 days), the prices ranged between seven daily wages if the substance came from a public pharmacy (lowest median price, 0.5USD/single dose) to 56 daily wages if the substance was bought from a private pharmacy (highest median price, 1.2USD) [56].
In this context, namely concerning the rising antibiotic consumption globally with high proportional Watch group use, promoting universal health coverage (UHC), and addressing economic inequalities that may force patients to choose treatment options based on affordability and accessibility rather than medical necessity and appropriateness is crucial to combat AMR.
Several limitations of the present study should be considered. No data on the antibiotic start date, full duration and prescriber (if any) were available and data on hospitalization was incomplete especially for CIV and DRC so it was not possible to clearly distinguish outpatient versus inpatient treatment in the ten days prior to study enrolment. Furthermore, ANDEMIA patients were enrolled within 24 h of presentation to the health care facility, so antibiotics received after enrolment were not captured. This limited the ability to conduct a regression analysis to further assess the factors influencing antimicrobial use. The reported antibiotic data including name and date of last dose may have also been affected by recall bias or lack of documentation during the clinical interview and completion of questionnaire. Differences in these practices or limitations may have also occurred across participating study facilities although all surveillance personal received the same training materials. Namely, in RSA, it was reported that surveillance officers allowed enrolment and specimen collection for up to 48 h, which may have led to higher reported antibiotic use and/or multiple therapies. Approximations concerning the routes of administration (i.e. parenteral, oral, or both/other) must be interpreted with caution, as the actual data were not reported and these results were coded according to the provided antibiotic names, available guidelines and expert clinical opinion. Also, antibiotic dosages were not available, which limits comparison with large antibiotic consumption studies based on sales data such as Klein et al. [5, 9]. It was not possible to assess reported antibiotic use in special patient populations such as those with human immunodeficiency virus (HIV) or tuberculosis (Tb) as patient numbers across health care facility locations were too small. Finally, severity of illness and patient follow-up were not assessed in this study as the complete data for these variables were not available.
Conclusion
Relatively high levels of Watch group antibiotic use, particularly in acute febrile disease of unknown cause and for gastrointestinal infections, pose a challenge to antibiotic use interventions to address the burden of AMR. A nuanced perspective on the clinical presentation of the patient, the country-context, accessibility, and affordability of care and treatment needs to be considered when planning and implementing strategies to reduce inappropriate Watch group antibiotic use.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1. Table of relevant country data and study sites (.pdf).
Additional file 2. Figure on ANDEMIA case-definitions (.pdf).
Additional file 3. Question on antibiotics use, ANDEMIA case investigation form (.pdf).
Additional file 4. Table of the coding frameworks for the antibiotic formulation and WHO AWaRe criteria (.pdf).
Additional file 5. Table: Body Mass Index calculation (pdf).
Additional file 6. Table of characteristics of ANDEMIA patients enrolled from 1 February 2018 till 26 May 2022 by country (.pdf).
Additional file 7. Table of reported antibiotic use in the ten days prior to study enrolment in the study population (.docx).
Additional file 8. Table of syndrome enrolment of patients that reported antibiotic use in the ten days prior to study enrolment with row frequencies (.docx).
Additional file 9. Figure on proportional antibiotic use according to WHO AWaRe classification by country, before and during COVID-19 pandemic (.docx/.tif).
Additional file 10. Figure on total reported antibiotics regardless of date of last dose among ANDEMIA total as well as by country (.pdf).
Additional file 11. Table on the number of different antibiotic substances reported in the ANDEMIA study by country and by location (.pdf).
Acknowledgements
We acknowledge the whole ANDEMIA study team and all the patients for their willingness to participate in this study. We are grateful to all medical and technical staff at the study sites for their engagement in patient enrolment and data collection. We thank Zoungrana Jacques and Sanogo Bintou from Centre Hospitalier Universitaire Sourô Sanou de Bobo-Dioulasso as well as Kalafong clinical staff Dr Nicolette M. du Plessis, Prof Theunis Avenant and Dr Maryke de Villiers for site management, identification of patients and specimen collection. We thank Essia Belarbi, Kathrin Nowak, Paul Pitzinger, Jan Walther, Suanne Köhler and Sarah Kribi (all RKI) for support in trainings on data collection, data management as well as logistic support.
Abbreviations
- AFDUC
Acute febrile disease of unknown cause
- AMR
Antimicrobial resistance
- ANDEMIA
African Network for Improved Diagnostics, Epidemiology and Management of Common Infectious Agents
- AWaRe
Access, Watch, Reserve
- BF
Burkina Faso
- BMI
Body Mass Index
- CAP
Community acquired pneumonia
- CI
Confidence Interval
- CIV
Côte d’Ivoire
- DRC
Democratic Republic of the Congo
- GCS
Glasgow Coma Scale
- GI
Gastrointestinal infection
- HIV
Human immunodeficiency virus
- IQR
Interquartile range
- LMICs
Low-and middle-income countries
- PPS
Point-prevalence survey
- RSA
Republic of South Africa
- RTI
Acute respiratory infection
- Tb
Tuberculosis
- USD
United States Dollar
- WaSH
Water, sanitation and hygiene
- WHO
World Health Organization
Author contributions
S.J., S.M., A.P., C.A., M.A., T.E., F.Ka., F.Kw., F.L., B.M., A.O., N.P., F.T., A.T., M.V., G.S. and S.T. contributed to the overall surveillance study protocol development and data collection.I.W., S.T., G.S., T.E. and M.A. formulated the secondary research question and relevant methodology. I.W., S.S., V.G., AC.V. and S.T. conducted the data cleaning and analysis.I.W. drafted the manuscript with substantial input of S.J., S.M., A.P., C.A., M.A., T.E., V.G., F.Ka., F.Kw., F.L., B.M., A.O., N.P., S.S., F.T., A.T., M.V., A.V., G.S. and S.T. All authors reviewed the manuscript. The final version of the manuscript and its submission for publication was agreed on by all authors.
Funding
Open Access funding enabled and organized by Projekt DEAL. The ANDEMIA study was funded by a grant from the German Federal Ministry of Education and Research (BMBF; grant number 01KA1606). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Additional modules in the study were financed by the German Federal Ministry of Health (BMG) within the Global Health Protection Programme (GHPP) ZMVI1-2517GHP703 TP08 und TP09. Also, this funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. Datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The ANDEMIA study protocol was approved by the investigators’ institutional ethics committees in all participating countries [(CIV: Comité National d’Ethique des Sciences de la Vie et de la Santé de Côte d’Ivoire (105/MSHP/CNER-dk); Burkina Faso: Comité d’Ethique pour la Recherche en Santé (2017–5–057); Democratic Republic of the Congo: Comité d’Ethique de l’Ecole de Santé Publique de l’Université de Kinshasa (ESP/CE/042/2017); South Africa: Faculty of Health Research Ethic Committee (Medical), University of Witwatersrand (M170403), Faculty of Health Sciences Research Ethics Committee, University of Pretoria (101/2017); Germany: Ethikkommission - Ethikausschuss am Campus Virchow-Klinikum, Charité (EA2/230/17)]. Written informed consent was obtained from all enrolled patients or their legal guardian. Patient privacy was protected by removing all personal identifiers before entry in the database, and access to patient level data was restricted to selected study personnel.
Consent for publication
Not applicable.
Competing interests
One author NP reported a potential competing interest as follows:NP has received contractual fees by GSK, has received grants or contracts from PATH Center for Vaccine Innovation and Access, has received support from the Defense Threat Reduction Agency for attending meetings for the Annual Biological Safety Conference, Milwaukee and has participated on the GSK Rotavirus Advisory Board. All the other authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629–55. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee A, Modarai M, Naylor NR, Boyd SE, Atun R, Barlow J, et al. Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis. 2018;18(12):e368–e78. doi: 10.1016/S1473-3099(18)30296-2. [DOI] [PubMed] [Google Scholar]
- 3.Roberts SC, Zembower TR. Global increases in antibiotic consumption: a concerning trend for WHO targets. Lancet Infect Dis. 2021;21(1):10–1. doi: 10.1016/S1473-3099(20)30456-4. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Action Plan on Antimicrobial Resistance. Geneva: 2015. Report No.: 978 92 4 150976 3 https://www.who.int/publications/i/item/9789241509763.
- 5.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci. 2018;115(15):E3463–E70. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Access, Watch, Reserve (AWaRe) classification of antibiotics for evaluation and monitoring of use. Geneva: World Health Organization; 2021. [Google Scholar]
- 7.Sharland M, Gandra S, Huttner B, Moja L, Pulcini C, Zeng M, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—the new 2019 essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis. 2019;19(12):1278–80. doi: 10.1016/S1473-3099(19)30532-8. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Adopt AWaRe: Handle antibiotics with care. Geneva.: 2019. https://adoptaware.org/.
- 9.Klein EY, Milkowska-Shibata M, Tseng KK, Sharland M, Gandra S, Pulcini C, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000-15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21(1):107–15. doi: 10.1016/S1473-3099(20)30332-7. [DOI] [PubMed] [Google Scholar]
- 10.Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396(10258):1204-22. [DOI] [PMC free article] [PubMed]
- 11.Knowles R, Sharland M, Hsia Y, Magrini N, Moja L, Siyam A, et al. Measuring antibiotic availability and use in 20 low- and middle-income countries. Bull World Health Organ. 2020;98(3):177–87c. doi: 10.2471/BLT.19.241349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendelson M, Rottingen JA, Gopinathan U, Hamer DH, Wertheim H, Basnyat B, et al. Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet (London England) 2016;387(10014):188–98. doi: 10.1016/S0140-6736(15)00547-4. [DOI] [PubMed] [Google Scholar]
- 13.Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen J-A, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. The Lancet. 2016;387(10014):168–75. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 14.Access to Medicine Foundation. Antimicrobial Resistance Benchmark 2021. 2021. https://accesstomedicinefoundation.org/.
- 15.World Health Organization. Antimicrobial resistance and primary health care. Geneva: World Health Organization: 2018. Contract No.: WHO/HIS/SDS/2018.56. https://apps.who.int/iris/handle/10665/326454.
- 16.Sulis G, Adam P, Nafade V, Gore G, Daniels B, Daftary A, et al. Antibiotic prescription practices in primary care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2020;17(6):e1003139. doi: 10.1371/journal.pmed.1003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denyer Willis L, Chandler C. Quick fix for care, productivity, hygiene and inequality: reframing the entrenched problem of antibiotic overuse. BMJ Global Health. 2019;4(4):e001590. doi: 10.1136/bmjgh-2019-001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Global Health. 2019;4(6):e002104. doi: 10.1136/bmjgh-2019-002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista AD, Figueiras DAR, Zapata-Cachafeiro A, Roque M, Herdeiro F. MT. Antibiotic dispensation without a prescription worldwide: a systematic review. Antibiot (Basel Switzerland). 2020;9(11). [DOI] [PMC free article] [PubMed]
- 20.Belachew SA, Hall L, Selvey LA. Non-prescription dispensing of antibiotic agents among community drug retail outlets in sub-saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2021;10(1):13. doi: 10.1186/s13756-020-00880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Do NTT, Vu HTL, Nguyen CTK, Punpuing S, Khan WA, Gyapong M, et al. Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. The Lancet Global Health. 2021;9(5):e610–e9. doi: 10.1016/S2214-109X(21)00024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert G, Achi V, Ahuka S, Belarbi E, Bourhaima O, Eckmanns T, et al. The African Network for Improved Diagnostics, Epidemiology and Management of common infectious agents. BMC Infect Dis. 2021;21(1):539. doi: 10.1186/s12879-021-06238-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Direction des Activités Pharmaceutiques Côte d’Ivoire. Liste nationale des médicaments essentiels et du matériel bio-médical (version 2020). 2020. http://www.leemafrique.org/fr/reglement.asp?num=10&select_zone=C%C3%B4te+d%27%27Ivoire&select_sujet=.
- 24.République Démocratique du Congo. Liste Nationale des Médicaments Essentiels 2018/2020. https://acorep-dpmrdc.org/publication/essentiels.
- 25.World Health Organization. Model List of Essential Medicines– 22nd List., 2021. Geneva: World Health Organization, 2021 (WHO/MHP/HPS/EML/2021.02). Licence: CC BY-NC-SA 3.0 IGO.: 2021 https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02.
- 26.South African National Department of Health. Standard Treatment Guidelines and Essential Medicines List for South Africa 2020 [cited 2023/03/05]. 7:[626]. Available from: https://www.knowledgehub.org.za/e-library.
- 27.World Health Organization. Child growth standards 2023 [cited 2022/10/01]. The WHO Anthro Survey Analyser, anthro tool for BMI z score for 0–5 year old. Available from: https://www.who.int/tools/child-growth-standards/software.
- 28.World Health Organization. Growth reference data for 5–19 years 2023 [cited 2022/10/22]. Stata anthroplus tool for BMI z score calculation for 5–19 years. Available from: https://www.who.int/tools/growth-reference-data-for-5to19-years/application-tools.
- 29.World Health Organization. A healthy lifestyle - WHO recommendations. Europe 2010 6 May 2010. Contract No.: 10.08.2022. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations.
- 30.Sulis G, Sayood S, Katukoori S, Bollam N, George I, Yaeger LH, et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(9):1193–202. doi: 10.1016/j.cmi.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Valia D, Ingelbeen B, Kaboré B, Karama I, Peeters M, Lompo P, et al. Use of WATCH antibiotics prior to presentation to the hospital in rural Burkina Faso. Antimicrob Resist Infect Control. 2022;11(1):59. doi: 10.1186/s13756-022-01098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingelbeen B, Koirala KD, Verdonck K, Barbé B, Mukendi D, Thong P, et al. Antibiotic use prior to seeking medical care in patients with persistent fever: a cross-sectional study in four low- and middle-income countries. Clin Microbiol Infection: Official Publication Eur Soc Clin Microbiol Infect Dis. 2021;27(9):1293–300. doi: 10.1016/j.cmi.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Hsia Y, Lee BR, Versporten A, Yang Y, Bielicki J, Jackson C, et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. The Lancet Global Health. 2019;7(7):e861–e71. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 34.Sonda TB, Horumpende PG, Kumburu HH, van Zwetselaar M, Mshana SE, Alifrangis M, et al. Ceftriaxone use in a tertiary care hospital in Kilimanjaro, Tanzania: a need for a hospital antibiotic stewardship programme. PLoS ONE. 2019;14(8):e0220261. doi: 10.1371/journal.pone.0220261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauwels I, Versporten A, Drapier N, Vlieghe E, Goossens H. Network tG-P. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother. 2021;76(6):1614–24. doi: 10.1093/jac/dkab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 Socio-Demographic Index 1950–2019. Seattle: Institute for Health Metrics and Evaluation: 2020. https://ghdx.healthdata.org/record/ihme-data/gbd-2019-socio-demographic-index-sdi-1950-2019.
- 37.Institute for Health Metrics and Evaluation, Compare GBD. University of Washington; 2019 [cited 2023/03/23]. Available from: https://vizhub.healthdata.org/gbd-compare/.
- 38.Katz ML, Mueller LV, Polyakov M, Weinstock SF. Where have all the antibiotic patents gone? Nat Biotechnol. 2006;24(12):1529–31. doi: 10.1038/nbt1206-1529. [DOI] [PubMed] [Google Scholar]
- 39.Zanichelli V, Sharland M, Cappello B, Moja L, Getahun H, Pessoa-Silva C, et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull World Health Organ. 2023;101(4):290–6. doi: 10.2471/BLT.22.288614. [DOI] [Google Scholar]
- 40.World Health Organization. The WHO AWaRe (Access, Watch, Reserve) antibiotic book. Geneva: World Health Organization: 2022. https://www.who.int/publications/i/item/9789240062382.
- 41.Mathibe LJ, Zwane NP. Unnecessary antimicrobial prescribing for upper respiratory tract infections in children in Pietermaritzburg, South Africa. Afr Health Sci. 2020;20(3):1133–42. doi: 10.4314/ahs.v20i3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeika EV, Ingelbeen B, Kemah BL, Wirsiy FS, Fomengia JN, van der Sande MAB. Comparative assessment of the prevalence, practices and factors associated with self-medication with antibiotics in Africa. Tropical medicine & international health: TM & IH; 2021. pp. 862–81. [DOI] [PubMed] [Google Scholar]
- 43.Adebisi YA, Jimoh ND, Ogunkola IO, Uwizeyimana T, Olayemi AH, Ukor NA, et al. The use of antibiotics in COVID-19 management: a rapid review of national treatment guidelines in 10 African countries. Trop Med Health. 2021;49(1):51. doi: 10.1186/s41182-021-00344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nandi A, Pecetta S, Bloom DE. Global antibiotic use during the COVID-19 pandemic: analysis of pharmaceutical sales data from 71 countries, 2020–2022. eClinicalMedicine. 2023;57. [DOI] [PMC free article] [PubMed]
- 45.King LM, Lovegrove MC, Shehab N, Tsay S, Budnitz DS, Geller AI, et al. Trends in US Outpatient Antibiotic prescriptions during the Coronavirus Disease 2019 Pandemic. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2021;73(3):e652–e60. doi: 10.1093/cid/ciaa1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harant A. Assessing transparency and accountability of national action plans on antimicrobial resistance in 15 African countries. Antimicrob Resist Infect Control. 2022;11(1):15. doi: 10.1186/s13756-021-01040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ministère de la santé Burkina Faso. Plan d’action multisectoriel de lutte contre la resistance aux antimicrobiens Octobre 2017– Septembre 2020. 2017. https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/burkina-faso_national-action-plan-amr_2017-2020-(french).pdf?sfvrsn=6bbec5fa_1&download=true
- 48.Republic of South Africa - Departments of Health and Agriculture Forestry and Fisheries. South African antimicrobial resistance national strategy framework; A One Health approach 2018–2024 2018 [cited 2023/06/06]. Available from: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/south-africa-antimicrobial-resistance-national-action-plan-2018---2024.pdf?sfvrsn=533118b0_1&download=true.
- 49.République Démocratique du Congo - Ministère de la santé publique. Plan national de lutte contre la résistance aux antimicrobiens. 2018 November 2018. https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/drc_nap_2018.pdf?sfvrsn=de9a715e_1&download=true.
- 50.Patel J, Harant A, Fernandes G, Mwamelo AJ, Hein W, Dekker D, et al. Measuring the global response to antimicrobial resistance, 2020–21: a systematic governance analysis of 114 countries. The Lancet Infectious Diseases; 2023. [DOI] [PubMed]
- 51.World Health Organization. Burkina Faso national action plan on antimicrobial resistance: review of progress in the human health sector. Geneva 2021. https://apps.who.int/iris/bitstream/handle/10665/354778/9789290313557-fre.pdf?sequence=1&isAllowed=y
- 52.Bawontuo V, Adomah-Afari A, Amoah WW, Kuupiel D, Agyepong IA. Rural healthcare providers coping with clinical care delivery challenges: lessons from three health centres in Ghana. BMC Fam Pract. 2021;22(1):32. doi: 10.1186/s12875-021-01379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vliegenthart-Jongbloed K, Jacobs J. Not recommended fixed-dose antibiotic combinations in low- and middle-income countries– the example of Tanzania. Antimicrob Resist Infect Control. 2023;12(1):37. doi: 10.1186/s13756-023-01238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EPA, Hamadani BHK, Zaraa S, et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health. 2021;5(12):e893–e904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schäfermann S, Neci R, Ndze EN, Nyaah F, Pondo VB, Heide L. Availability, prices and affordability of selected antibiotics and medicines against non-communicable diseases in western Cameroon and northeast DR Congo. PLoS ONE. 2020;15(1):e0227515. doi: 10.1371/journal.pone.0227515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutema G, Engidawork E. Affordability of commonly prescribed antibiotics in a large tertiary teaching hospital in Ethiopia: a challenge for the national drug policy objective. BMC Res Notes. 2018;11(1):925. doi: 10.1186/s13104-018-4021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table of relevant country data and study sites (.pdf).
Additional file 2. Figure on ANDEMIA case-definitions (.pdf).
Additional file 3. Question on antibiotics use, ANDEMIA case investigation form (.pdf).
Additional file 4. Table of the coding frameworks for the antibiotic formulation and WHO AWaRe criteria (.pdf).
Additional file 5. Table: Body Mass Index calculation (pdf).
Additional file 6. Table of characteristics of ANDEMIA patients enrolled from 1 February 2018 till 26 May 2022 by country (.pdf).
Additional file 7. Table of reported antibiotic use in the ten days prior to study enrolment in the study population (.docx).
Additional file 8. Table of syndrome enrolment of patients that reported antibiotic use in the ten days prior to study enrolment with row frequencies (.docx).
Additional file 9. Figure on proportional antibiotic use according to WHO AWaRe classification by country, before and during COVID-19 pandemic (.docx/.tif).
Additional file 10. Figure on total reported antibiotics regardless of date of last dose among ANDEMIA total as well as by country (.pdf).
Additional file 11. Table on the number of different antibiotic substances reported in the ANDEMIA study by country and by location (.pdf).
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. Datasets used during the current study are available from the corresponding author on reasonable request.