Abstract
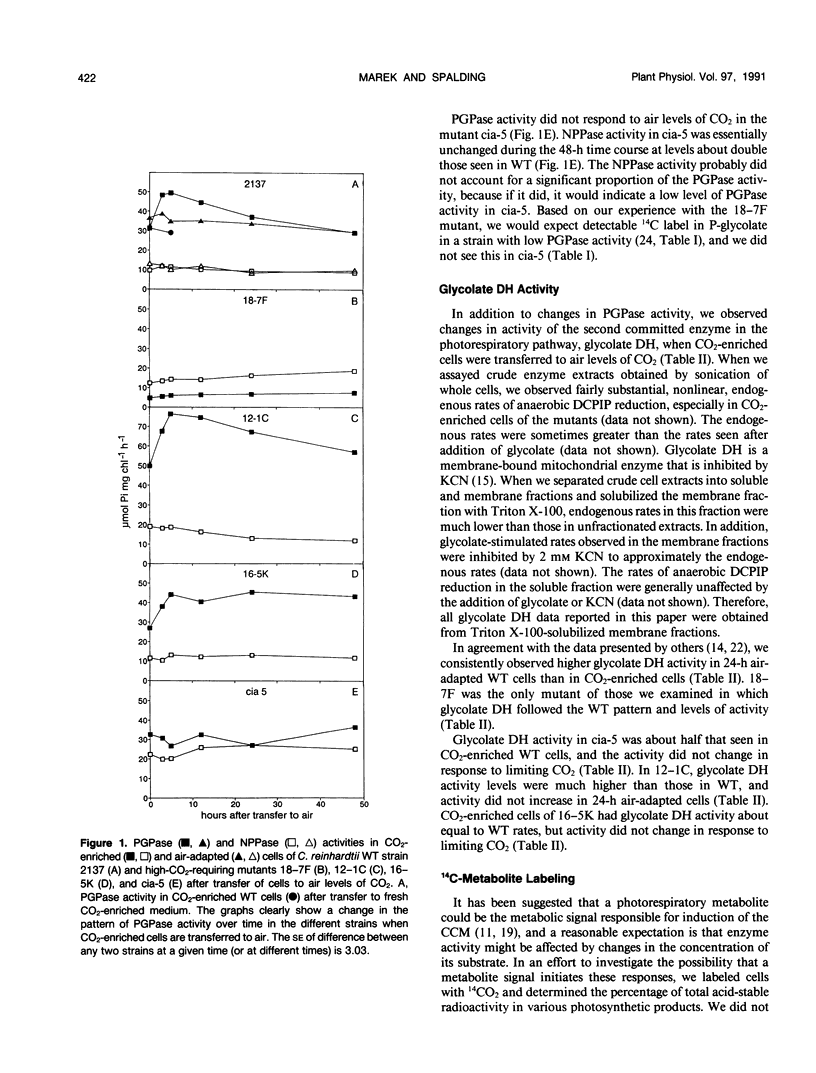
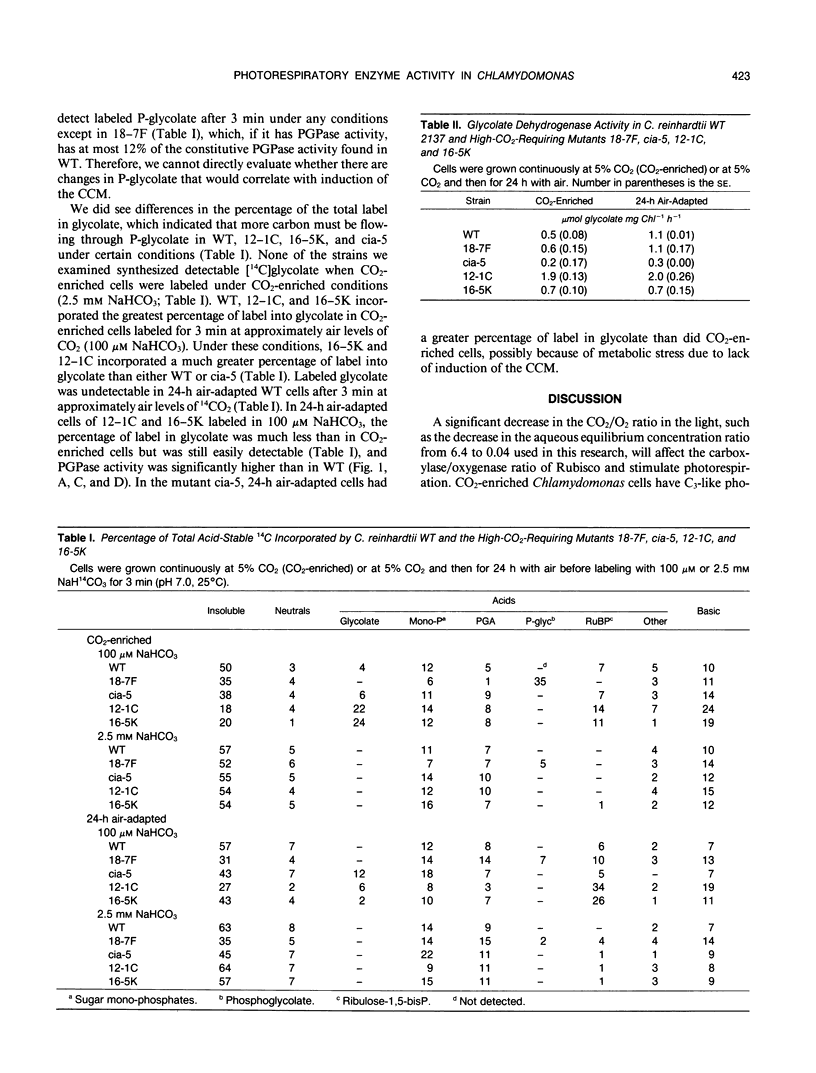
The activity of two photorespiratory enzymes, phosphoglycolate phosphatase (PGPase) and glycolate dehydrogenase (glycolate DH), changes when CO2-enriched wild-type (WT) Chlamydomonas reinhardtii cells are transferred to air levels of CO2. Adaptation to air levels of CO2 by Chlamydomonas involves induction of a CO2-concentrating mechanism (CCM) which increases the internal inorganic carbon concentration and suppresses oxygenase activity of ribulose-1,5-bisphosphate carboxylase/oxygenase. PGPase in cell extracts shows a transient increase in activity that reaches a maximum 3 to 5 hours after transfer and then declines to the original level within 48 hours. The decline in PGPase activity begins at about the time that physiological evidence indicates the CCM is approaching maximal activity. Glycolate DH activity in 24 hour air-adapted WT cells is double that seen in CO2-enriched cells. Unlike WT, the high-CO2-requiring mutant, cia-5, does not respond to limiting CO2 conditions: it does not induce any known aspects of the CCM and it does not show changes in PGPase or glycolate DH activities. Other known mutants of the CCM show patterns of PGPase and glycolate DH activity after transfer to limiting CO2 which are different from WT and cia-5 but which are consistent with changes in activity being initiated by the same factor that induces the CCM, although secondary regulation must also be involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beezley B. B., Gruber P. J., Frederick S. E. Cytochemical localization of glycolate dehydrogenase in mitochondria of chlamydomonas. Plant Physiol. 1976 Sep;58(3):315–319. doi: 10.1104/pp.58.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Gruber P. J., Tolbert N. E. The occurrence of glycolate dehydrogenase and glycolate oxidase in green plants: an evolutionary survey. Plant Physiol. 1973 Oct;52(4):318–323. doi: 10.1104/pp.52.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husic H. D., Tolbert N. E. Properties of Phosphoglycolate Phosphatase from Chlamydomonas reinhardtii and Anacystis nidulans. Plant Physiol. 1985 Oct;79(2):394–399. doi: 10.1104/pp.79.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Berry J. A. Glycolate Excretion and the Oxygen to Carbon Dioxide Net Exchange Ratio during Photosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 1981 Feb;67(2):229–232. doi: 10.1104/pp.67.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Harel E., Kaplan A. Adaptation of the Cyanobacterium Anabaena variabilis to Low CO(2) Concentration in Their Environment. Plant Physiol. 1983 Jan;71(1):208–210. doi: 10.1104/pp.71.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Husic H. D., Tolbert N. E., Kitayama M., Manuel L. J., Togasaki R. K. Isolation and Characterization of a Mutant of Chlamydomonas reinhardtii Deficient in the CO(2) Concentrating Mechanism. Plant Physiol. 1989 Mar;89(3):897–903. doi: 10.1104/pp.89.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Wilson B. J., Tolbert N. E. Glycolate Metabolism and Excretion by Chlamydomonas reinhardtii. Plant Physiol. 1986 Nov;82(3):821–826. doi: 10.1104/pp.82.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Spalding M. H., Jeffrey M. Membrane-Associated Polypeptides Induced in Chlamydomonas by Limiting CO(2) Concentrations. Plant Physiol. 1989 Jan;89(1):133–137. doi: 10.1104/pp.89.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Reduced Inorganic Carbon Transport in a CO(2)-Requiring Mutant of Chlamydomonas reinhardii. Plant Physiol. 1983 Oct;73(2):273–276. doi: 10.1104/pp.73.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K. G., Togasaki R. K. Limitations on the Utilization of Glycolate by Chlamydomonas reinhardtii. Plant Physiol. 1981 Jul;68(1):28–32. doi: 10.1104/pp.68.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer R. J., Mets L. Photosynthesis-deficient Mutants of Chlamydomonas reinhardii with Associated Light-sensitive Phenotypes. Plant Physiol. 1981 Mar;67(3):565–569. doi: 10.1104/pp.67.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Marek L. F., Spalding M. H. A Photorespiratory Mutant of Chlamydomonas reinhardtii. Plant Physiol. 1990 May;93(1):231–237. doi: 10.1104/pp.93.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Spalding M. H. Adaptation of Chlamydomonas reinhardtii High-CO(2)-Requiring Mutants to Limiting CO(2). Plant Physiol. 1989 Jul;90(3):1195–1200. doi: 10.1104/pp.90.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


