Abstract
Recent evidence suggests that alcohol use disorder (AUD) may manifest itself differently in women compared to men. Women experience AUDs on an accelerated timeline and may have certain regional vulnerabilities. In male rats, neuronal cell death and astrocyte reactivity are noted following induction of alcohol dependence in an animal model of AUD. However, the regional and temporal patterns of neurodegeneration and astrocyte reactivity have yet to be fully examined in females using this model. Therefore, adult female rats were exposed to a 4-day binge model of alcohol dependence followed by several different periods of abstinence. Immunohistochemical markers for FluoroJade B, a label of degenerating neurons, and vimentin, a marker for reactive astrocytes, were utilized. The expression of these markers in cortical and limbic regions was quantified immediately after their last dose (e.g., T0), or 2, 7, and 14 days later. Significant neuronal cell death was noted in the entorhinal cortex and the hippocampus, similar to previous reports in males, and also several cortical regions not previously observed. Vimentin immunoreactivity was noted in the same regions as previously reported, in addition to three novel regions. Vimentin immunoreactivity also occurred both at earlier and later time points in some cortical and hippocampal regions. These data suggest that both neuronal cell death and vimentin immunoreactivity, sensitive markers of astrocyte reactivity, could be more widespread in females compared to males. Therefore, this study provides a framework for specific regions and time points which should be examined in future studies of alcohol-induced damage that include female rats.
Keywords: ethanol, vimentin, sex differences, alcohol use disorder, gliosis, GFAP
Introduction
The pervasiveness of alcohol misuse is startling; in the United States, nearly one-third of individuals will develop an Alcohol Use Disorder (AUD) at some point in their life, with about 14% of individuals meeting the criteria for an AUD in any given year (Grant et al., 2015). Although rates of AUD have historically been higher among men than women, this gap is shrinking, with an 84% increase in AUD in women compared to 35% in men in recent years (Grant et al., 2017). In lockstep with shifting societal norms, increasing numbers of women have adopted harmful patterns of alcohol use, such as binge drinking (Grucza et al., 2018; Keyes et al., 2008, 2011; Slade et al., 2016). Heavy alcohol use can result in brain volume reduction (Agartz et al., 1999; Pfefferbaum et al., 1992), neuronal cell death (Kril et al., 1997), and astrocyte reactivity (Franke, 1995; Miguel-Hidalgo, 2021), which may contribute to long-term cognitive deficits (Eckardt & Martin, 1986; Sullivan & Pfefferbaum, 2005). Despite initial evidence that females may be more sensitive to alcohol-induced brain damage than males (Hommer et al., 2001), few studies have sought to characterize how neuronal cell death and astrocyte reactivity may occur differently in these two populations.
Research on how AUD related cognitive deficits and neurotoxicity may differ between men and women have produced contradictory results. Initial studies indicated that compared to men, women with AUDs may experience alcohol-related medical problems on an accelerated timeline (Ashley et al., 1977; Mumenthaler et al., 1999; Piazza et al., 1989), develop cognitive and motor deficits with shorter drinking histories (Acker, 1985), and perform worse on some cognitive tests (Acker, 1986; Sullivan et al., 2002). Neuroimaging studies have sought to tie these behaviors to structural deficits, with mixed results (Cortez et al., 2020; Fama et al., 2020; Verplaetse et al., 2021). Some magnetic resonance imaging (MRI) studies have revealed that women are more susceptible to alcohol-related structural abnormalities than men, especially in corticolimbic regions (Agartz et al., 2003; Hommer et al., 2001; Mann et al., 2005; Pfefferbaum et al., 2001). Others indicate greater brain volume loss in men compared to women, not just in corticolimbic regions (Momenan et al., 2012; Sawyer et al., 2017) but in the cerebellum as well (Sawyer et al., 2016; Sullivan et al., 2010). Recent MRI studies with modern voxel based imaging techniques do not find sex differences in brain volumes of those with AUD (Demirakca et al., 2011; Mechtcheriakov et al., 2007). Although some evidence exists for sex differences, more than 80% of recent alcohol related neuroimaging studies either do not analyze by sex or are underpowered to do so, making interpretations of the human imaging literature challenging (Verplaetse et al., 2021).
Animal studies, which provide a means to directly investigate the causal effects of alcohol on brain structure and cognition, have yet to fully characterize neurodegeneration in females. Most studies of alcohol-induced brain damage utilize the Majchrowicz model of alcohol dependence (Majchrowicz, 1975), which emulates the binge-like intoxication, dependence, withdrawal, and damage observed in persons with an AUD. In male rats, this model results in neuronal cell death in multiple regions of the brain, most extensively in corticolimbic regions such as the hippocampus and rhinal and piriform cortices (Collins et al., 1996; Crews et al., 2000). Damage begins while the animals are intoxicated, peaking just after the last dose of alcohol and continuing at low but detectable levels for up to a week of abstinence from alcohol (Collins et al., 1996; Crews et al., 2000; Hayes et al., 2013; Kelso et al., 2011; Obernier, Bouldin, et al., 2002). However, few such studies have been performed in females. In those that have, one study found neuronal cell death in similar brain regions compared to previous studies with males, but they only focused on these common sites of damage (Leasure & Nixon, 2010). Another found a greater number of degenerating neurons in the hippocampus of females compared to males (Maynard et al., 2018), again focusing on specific regions where damage had been observed previously. In both of these studies, limited time points were examined and/or only regions where damage was previously observed in male rats were examined.
Another facet of alcohol-induced neuropathology, astrocyte reactivity, may also occur differently in women compared to men. Astrocytes are key regulators of brain homeostasis and perform important functions such as removing neurotransmitters from the synaptic space, ion buffering, vascular coupling, and modulating immune signaling (Giovannoni & Quintana, 2020; Sofroniew & Vinters, 2010; Verkhratsky & Nedergaard, 2018). With insult or disease, astrocytes become reactive, meaning they change phenotypically and reflect a host of morphological, molecular, and functional differences (Escartin et al., 2019, 2021). Reactive astrocytosis has been observed in numerous neuropathologies, including stroke (Li et al., 2008), Alzheimer’s Disease (Chun & Lee, 2018), and alcohol use (Franke, 1995; Sarkisyan et al., 2017). Though classically defined by the upregulation of intermediate filaments like GFAP and vimentin (Pekny & Nilsson, 2005), astrocyte reactivity also results in a variety of functional changes which may reflect the heterogenous phenotypes that result from each type of insult. For instance, neuroinflammation can produce reactive astrocytes capable of inducing neuronal cell death, while ischemia can produce a neuroprotective reactive phenotype (Liddelow et al., 2017). Therefore, in AUD, reactive astrocytes could play a functional role in both alcohol related neurodegeneration as well as recovery. The release of inflammatory mediators by reactive astrocytes (Blanco et al., 2004) may influence alcohol-induced neuronal cell death (Crews & Nixon, 2009). Vimentin, an intermediate filament protein expressed in low levels in astrocytes at baseline (Schnitzer et al., 1981), is a good tool to characterize reactive astrocytes (Kelso et al., 2011). For example, in animal models of traumatic brain injury (TBI), vimentin appears to be more specific than GFAP in identifying regions of necrosis or permanent ischemic injury (Petito et al., 1990; Schmidt-Kastner et al., 1990). It has similarly been used to suggest additional brain regions of vulnerability to alcohol in male rats (Kelso et al., 2011; Hayes et al., 2013) but has only been examined in female rats in one study using a once per week binge-like exposure model ((Hayes et al., 2013; Kelso et al., 2011; West et al., 2021). Vimentin expression has yet to be examined in female rats in a model of alcohol dependence.
Therefore, to understand the timeline of neurodegeneration and to perform an unbiased survey of areas of ethanol-induced neurotoxicity in females, we examined two measures in a time course following alcohol dependence. First, we utilized FluoroJade B (FJB) staining, which binds to dying neurons, to document the pattern of cell death in females during alcohol dependence, and across the first week of abstinence following ethanol. Then, immunohistochemistry for vimentin was employed to identify regions of astrocyte reactivity during these same time points.
Experimental Procedures
Animals
Adult female Sprague-Dawley rats (234 ± 17g and ~70 days old; Charles River Laboratories, Raleigh, NC, United States) were used in this study. A total of 86 rats were used across two institutions, 32 rats at the University of Kentucky (UK; rats in the T0 and T7 time points) and 54 rats at The University of Texas at Austin (UT; rats in T2, T7-colabel, and T14 groups), all while following identical experimental procedures including some overlap in personnel (Morris, Kelso, et al., 2010). Further, only the animal work and brain tissue extraction were performed at UK; all other work, notably all histology and immunohistochemistry, was conducted at UT. Rats were allowed to acclimate for 3 days to the environment of the respective vivariums and double-housed in standard polycarbonate cages except for a 24-hour period when animals were single housed to observe withdrawal behaviors. Rats were given free access to rat chow (UK = Teklad 2018 diet, Envigo, Madison, WI, United States; UT = Prolab® RMH 1800 5LL2∗, LabDiet, St Louis, MO, United States) except for during the ethanol administration procedure, and always had access to water. The different standard diets between the two institutions do not appear to have impacted any outcome measured to date. The vivariums were kept on a 12-hour light-dark cycle, with lights on from 0700 h. For three days before the start of the ethanol administration, rats were handled for 3 minutes each. All experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and The University of Texas at Austin Institutional Animal Care and Use Committee and followed the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Ethanol Administration Model
Rats were administered ethanol via intragastric gavage in a binge model of an AUD (Majchrowicz, 1975; Morris, Eaves, et al., 2010). An ethanol-containing diet (25% ethanol w/v in Vanilla Ensure Plus®, Abbott Labs, Columbus, OH, United States) was administered 3 times per day (every 8 hours) for four days (Figure 1A). An initial dose of 5.0 g/kg was administered with subsequent doses administered based on the intoxication behavior of each rat (Figure 1B). Control rats received an isocaloric diet (Vanilla Ensure Plus® containing dextrose). The amount of diet received by control rats was calculated as the average volume of the diet received by ethanol-treated rats at each administration time point. Blood ethanol concentrations (BEC) were determined via tail blood taken 90 minutes following the 7th dose of the ethanol administration procedure. Tail blood was taken via heparinized capillary tubes and stored in microcentrifuge tubes with 3μl of heparin (Meitheal Pharmaceuticals, Chicago, IL, United States), centrifuged for 5 minutes at 6500 rpm, and the plasma was stored at −20°C. BECs were analyzed with an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA, United States). Withdrawal behaviors were measured by observing rats every 30 minutes, beginning 10 hours after the final dose of alcohol for 17 hours. Behaviors were scored based on a withdrawal severity scale (Figure 1C; see also (Morris, Kelso, et al., 2010)).
Figure 1:
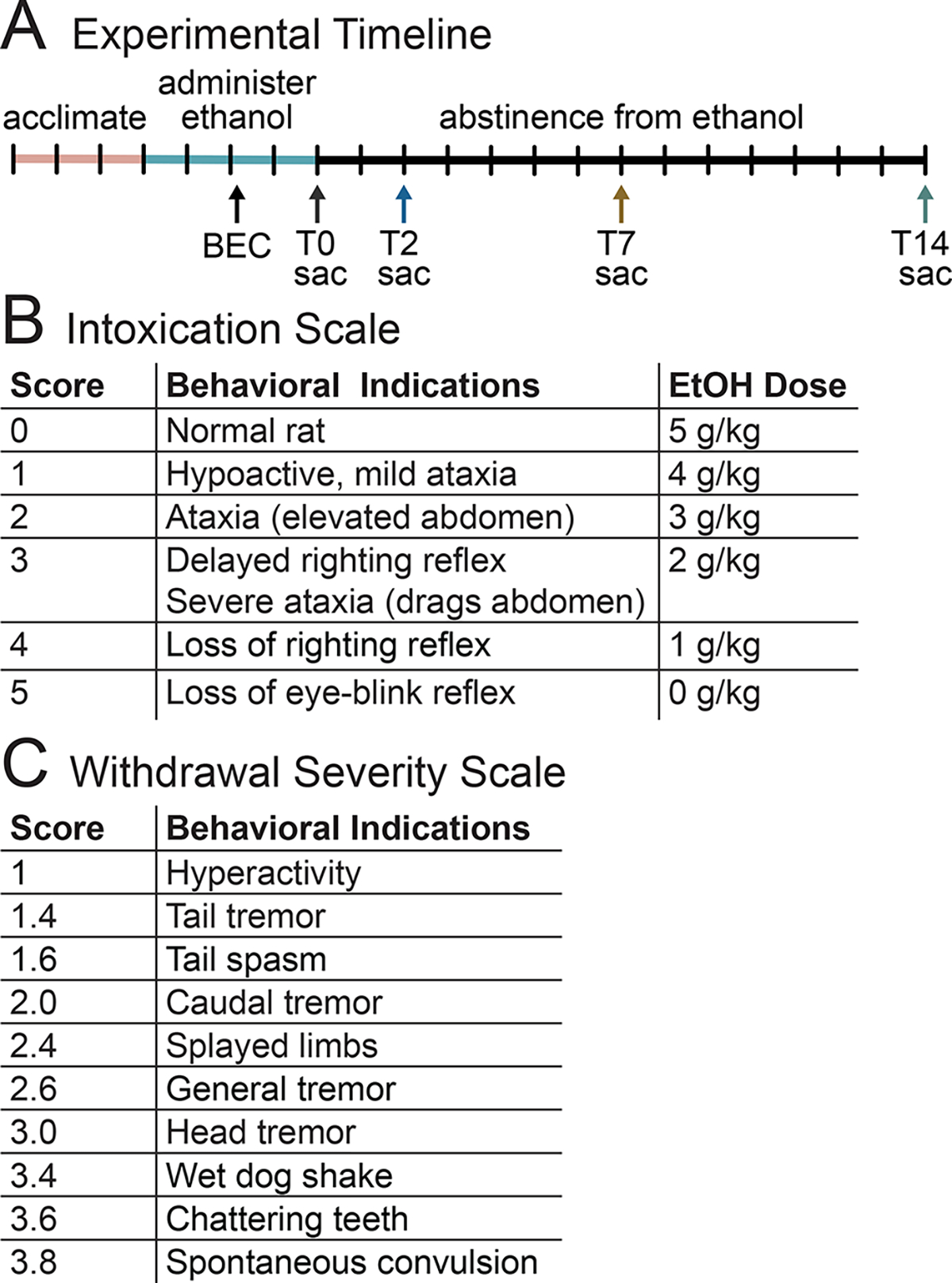
(A) Animals were acclimated to the vivarium, then administered ethanol three times a day for four days, with BECs taken after the 7th dose. Animals were sacrificed (sac) at one of four time points, either after the end of ethanol administration (T0), or at two (T2), seven (T7), or fourteen (T14) days of abstinence from ethanol. (B) Intoxication behavior scale and each corresponding ethanol dose. (C) Withdrawal severity scale used from 10 – 27 hours after the last dose of ethanol.
Tissue Preparation
As prior work in male rats indicates that FJB staining occurs maximally directly following the last dose (T0) while vimentin immunoreactivity peaks following 2 to 7 days of abstinence (Kelso et al., 2011), rats were sacrificed at 0, 2, 7, and 14 days after ethanol exposure. At each time point, rats were given a lethal dose of sodium pentobarbital (i.p.; Fatal-Plus®, Vortech Pharmaceuticals, Dearborn, MI, United States) and perfused transcardially with 0.1M phosphate buffered saline (PBS, pH=7.4) followed by 4% paraformaldehyde (PFA). Brains were extracted and post-fixed overnight in 4% PFA, then rinsed and stored in PBS at 4°C. Brain tissue was sectioned in twelve series of 40μm sections using a vibrating microtome (Leica VT100S, Wetzlar, Germany) starting randomly at approximately 2.4mm through 7.0mm Bregma. Tissue was stored in 24-well plates with cryoprotectant at −20°C.
FluoroJade B Staining
FluoroJade B (FJB) is a fluorescent dye that has been shown previously to identify damaged neurons (Schmued & Hopkins, 2000) and with better signal to noise resolution in our hands than the newer FluoroJade C (Schmued et al., 2005). Although the stain does not provide information on the mode of cell death, past studies indicate that cell death is more necrotic in nature in this model of alcohol dependence (Morris, Eaves, et al., 2010; Obernier, Bouldin, et al., 2002). Every 12th section of each brain (adjacent sections to vimentin) as well as a positive control (rat stroke brain) were mounted on Superfrost Plus® slides (Fisher Scientific) and allowed to dry overnight. The next morning, slides were placed on a slide warmer at 24°C for an additional 3 hours before staining. The sections were processed through 1% NaOH in 80% EtOH for 5 minutes, 70% EtOH for 2 minutes, ddH2O for 2 minutes, and then 0.06% KMnO4 for 10 minutes. Following a wash in ddH2O, the slides were incubated in the dark with FJB (MilliporeSigma) for 20 minutes before the final three ddH2O washes for 1 minute each. Finally, the tissue was dried on a slide warmer in the dark at 24°C for 30 minutes and then coverslipped in Cytoseal®.
Vimentin Immunohistochemistry
Immunohistochemistry was utilized to visualize vimentin, an intermediate filament protein expressed in some populations of astrocytes (Schnitzer et al., 1981). Every 12th section (a single well) was processed for free-floating immunohistochemistry as previously described (Kelso et al., 2011). Importantly, dilution curves were conducted to determine antibody dilutions and included negative controls (no primary; no secondary) to revalidate all methodology in the new lab location. Sections were first rinsed (3 × 5 min) in tris-buffered saline (TBS), then incubated in a 0.6% peroxidase solution for 30 minutes. Following more rinses of TBS, tissue was blocked (3% horse serum/ 0.1% Triton-X/ TBS), then incubated in anti-vimentin primary antibody (Chemicon, 1:750; determined by dilution curve) in blocking solution overnight at 4°C. Sections were washed 3 times for 10 minutes in blocking solution, then incubated in horse anti-mouse, rat adsorbed biotinylated secondary antibody (Vector Laboratories, 1:200). Slices were washed in TBS, followed by avidin-biotin-peroxidase complex (ABC Elite kit; Vector Laboratories), rinsed in TBS again, and peroxidases were detected and visualized by diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA, United States). Sections were rinsed in TBS, mounted on slides, and coverslipped with Cytoseal® (Thermo Fisher Scientific, Waltham, MA, United States)
For the Vimentin and GFAP/Iba-1 co-immunofluorescent immunohistochemistry, sections were rinsed in TBS, blocked (10% goat serum/ 0.1% Triton-X/ TBS), and incubated overnight at 4°C in blocking solution with rabbit anti-GFAP (DAKO, 1:2500) or rabbit anti-Iba-1 (Wako, 1:400) and mouse anti-vimentin (Chemicon, 1:400). Sections were rinsed in blocking solution, and then incubated in secondary antibodies: Alexa Fluor 555 anti-mouse (Invitrogen, 1:200) and Alexa-Fluor 488 anti-rabbit (Invitrogen, 1:200). Slices were rinsed in TBS, mounted on slides, and coverslipped with ProLong Gold Anti-fade mounting medium (Invitrogen).
Quantification
FluoroJade B
FJB-stained slides were coded, and each section from 1.92mm Bregma to −7.44mm Bregma was closely examined for FJB-positive (FJB+) cells. The brain regions which contained FJB+ cells were identified by visual comparison of each brain slice with a rat brain atlas (George Paxinos & Charles Watson, 2009). FJB+ cells were manually counted across each section with a BX-51 Olympus microscope (Olympus, Center Valley, PA, United States) under blue light excitation (includes 488nm) at 10X and up to 80X magnifications as needed for each region. Manual profile counts were utilized as with previous (Kelso et al., 2011; Leasure & Nixon, 2010) due to the lack of background staining necessary for thickness estimation or regional demarcation, low numbers of profiles per region, and the heterogeneous labeling pattern (Noori & Fornal, 2011). FJB+ cells were counted by brain region in which significant staining was observed.
Vimentin immunohistochemistry
Vimentin-stained slides were coded, and each section from 1.92mm Bregma to −7.44mm Bregma was closely examined for vimentin-positive cells. Areas of significant vimentin+ cells with a glial morphology were analyzed. A BX-51 microscope under brightfield illumination was used to take 4x images in cortical and limbic regions (Stereo Investigator®; MBF Biosciences, Williston, VT, United States). Each image was quantified for percent area staining using Fiji (Schindelin et al., 2012) in a method similar to that used to analyze GFAP as reported by others (Miguel-Hidalgo et al., 2000, 2010). As Vimentin may faintly label endothelial cells (Baldwin & Scheff, 1996) in addition to astrocytes and radial glia, an area of each section containing only endothelial cells was quantified and subtracted from the analyzed region of interest. For co-labeling assessments of vimentin and GFAP or Iba-1, 60x z-stack images were taken with an Olympus FV300 laser scanning confocal microscope. Imaris software (Oxford Instruments, Tubney Woods, United Kingdom) was utilized to measure colocalization via the Coloc tool.
Statistical Analysis
All data were compiled in Microsoft Excel for Mac (Version 16.62) and analyzed with GraphPad Prism 9 (GraphPad Software, La Jolla, CA, United States). As all the regions in which FJB was found, and all regions except one where vimentin was quantified contained no cells of interest in control subjects, control subjects across the different time points were collapsed and data were analyzed using one-way ANOVA followed by Dunnet’s posthoc test. As F-tests revealed non-homogenous variance between groups, data were log-transformed before analysis (zeros were replaced with 0.00000001). The granule cell layer of the dentate gyrus contained vimentin immunoreactive radial glial cells in control subjects, as expected, therefore these data were analyzed by two-way ANOVA followed by Bonferroni posthoc test. Co-localization data were analyzed via one-way ANOVA followed by Tukey’s posthoc test. Any p<0.05 was considered a significant difference. All data are plotted using bar graphs of mean ± SEM including individual data points.
Results
Rat Model of an AUD - Intoxication and Dependence
Ethanol intoxication parameters resulting from the binge model of an AUD are outlined in Table 1. Overall, ethanol-treated rats achieved an intoxication score of 1.6 ± 0.4, which resulted in an ethanol dose of 9.6 ± 1.3 g/kg. BECs taken after the 7th ethanol dose measured 379.9 ± 82.0 mg/dL. The mean withdrawal score (mean of the highest scoring behavior each hour over the 17 hours) was 1.1 ± 1.0, while the peak withdrawal score (highest/most severe withdrawal behavior observed) was 2.7 ± 1.0. While dose and mean withdrawal parameters differed slightly but significantly among a couple of time points, the critical indicator, BEC, did not differ between groups. Furthermore, all parameters were similar to past studies with male and female rats utilizing this model (Barton et al., 2017; Kelso et al., 2011; Leasure & Nixon, 2010; Nawarawong et al., 2021).
Table 1.
Alcohol model subject data
| Time point | Subjects | BEC (mg/dL) | Dose (g/kg/day) | Intoxication Score | Mean WD | Peak WD |
|---|---|---|---|---|---|---|
| T0 | n=8 | 395.7±70.5 | 9.6±1.2 | 1.8±0.4b | n/a | n/a |
| T2 | n=11 | 412.6±62.4 | 8.5±0.8a | 1.5±0.4 | 2.0±1.0a | 2.5±1.0 |
| T7 | n=8 | 396.7±68.2 | 10.2±1.0 | 1.6±0.3 | 1.0±0.7 | 3.3±0.4 |
| T7 (co-label only) | n=8 | 362.2±45.1 | 9.9±1.2 | 1.5±0.2 | 0.7±0.5c | 2.7±1.1 |
| T14 | n=8 | 320.2±128 | 10.2±1.5 | 1.3±0.3 | 0.5±0.5 | 2.6±1.3 |
p < 0.05 T2 vs. T7/T14;
p < 0.05 T0 vs. T14;
p < 0.05 T2 vs. T7 co-label
Alcohol Increased FJB+ Cells in the Hippocampus and Cortical Regions
All sections of each brain were carefully surveyed for FJB+ cells. In controls, FJB was almost nonexistent, in line with previous studies (Hayes et al., 2013; Kelso et al., 2011; Leasure & Nixon, 2010). In ethanol-treated animals, 11 brain regions were found to contain at least some FJB+ cells: the insular cortex, piriform/amygdalopiriform transition area (apiriform) cortex, granule cell layer of the dentate gyrus, posterolateral cortical amygdaloid nucleus, cingulate/retrosplenial cortex, motor cortex, somatosensory cortex, parietal cortex, auditory cortex, visual cortex, and the peri/entorhinal cortex. In the granule cell layer, one-way ANOVA revealed an effect of treatment (Figure 2; F4,65=36.08, p≤0.0001), and Dunnet’s post hoc test showed that there was an increase at the T0 (p<0.0001), T2 (p<0.0001), and T7 (p=0.0002) time points. All remaining regions, except for the auditory cortex, had a statistically significant elevation of FJB+ cells in the ethanol group compared to controls (Figure 3): the cingulate/retrosplenial cortex (Figure 3; F4,65=4.282, p=0.0039) at the T2 (p=0.0037) time point; the insular cortex (Figure 3; F4,65=46.87, p<0.0001) at the T0 (p<0.0001), T2 (p<0.0001), and T7 (p<0.0001) time points; the motor cortex (Figure 3; F4,65=3.528, p=0.0115) at the T2 (p=0.0041) time point; the parietal cortex (Figure 3; F4,65=5.593, p=0.0006) at the T2 (p=0.0006) and T7 (p=0.0329) time points; the peri/entorhinal cortex (F4,65=58.12, p<0.0001) at the T0 (p<0.0001), T2 (p<0.0001), and T7 (p<0.0001) time points; the piriform/apiriform cortex (F4,65=38.55, p<0.0001) at the T0 (p<0.0001), T2 (p<0.0001), and T7 (p<0.0001) time points; the posterolateral cortical amygdaloid nucleus (F4,65=20.52, p<0.0001) at the T0 (p<0.0001), T2 (p<0.0001), and T7 (p<0.0001) time points; the somatosensory cortex (F4,65=8.506, p<0.0001) at T2 (p<0.0001); and the visual cortex (F4,65=5.681, p=0.0006) at T2 (p=0.0020) and T7 (p=0.0033).
Figure 2:
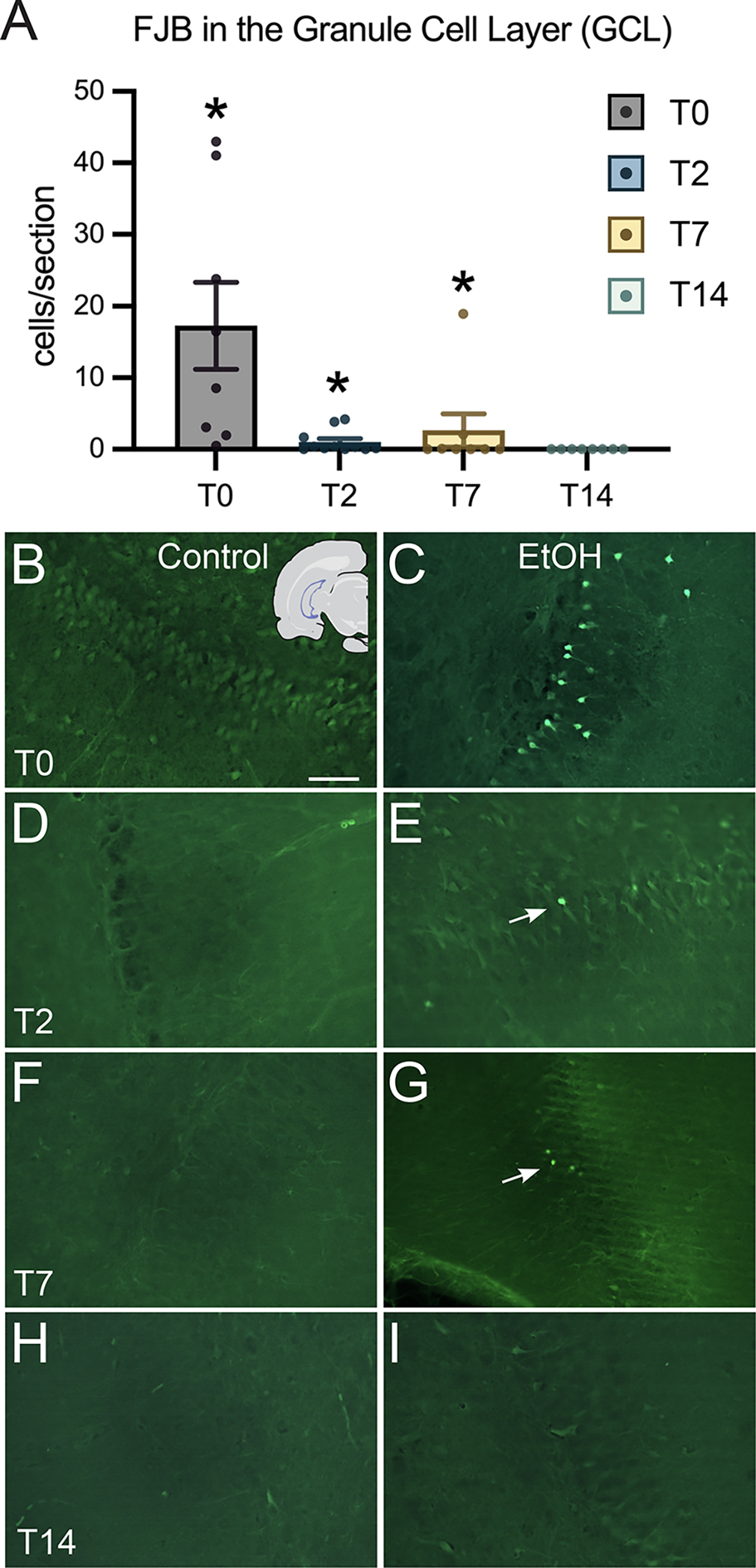
FluoroJade-B dye labeling of degenerating neurons in the granule cell layer of the dentate gyrus. (A) Quantification of FJB cells per section at each of the four time points in the dentate gyrus. (B-I) Representative images of the granule cell layer from control and ethanol treated rats are shown for each time point. Scale bar = 50μm; *p < 0.05.
Figure 3:
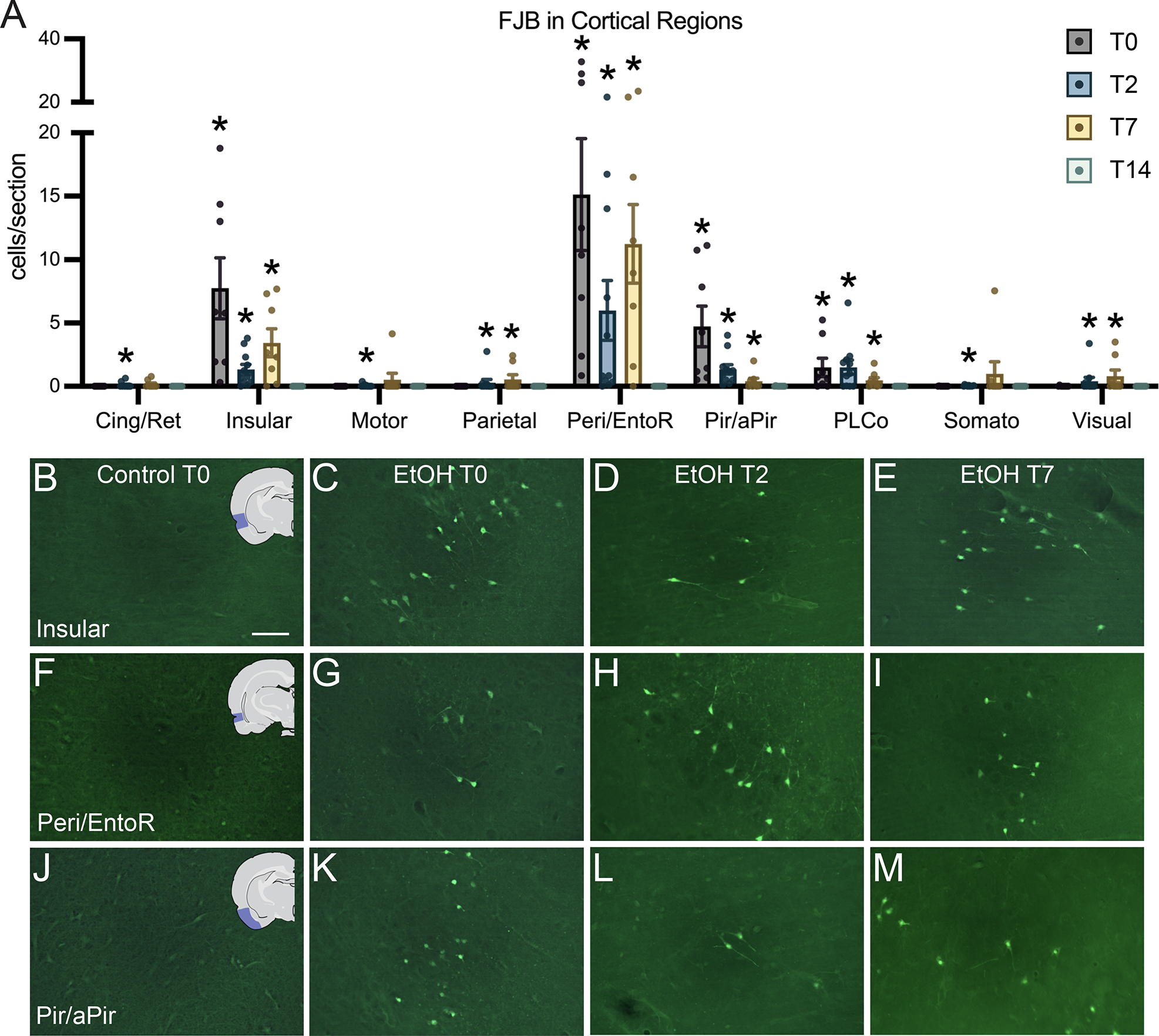
FluoroJade-B dye labeling of degenerating neurons in cortical regions. (A) Quantification of FJB cells per section in regions where FJB was significantly elevated above controls: cingulate/retrosplenial cortex (Cing/Ret), insular cortex, motor cortex, parietal cortex, combined perirhinal and entorhinal cortex (Peri/EntoR), combined piriform and apiriform cortex (Pir/aPir), posterolateral cortical amygdaloid nucleus (PLCo), somatosensory cortex, and visual cortex. (B-M) Representative images in control rats at T0 and ethanol treated rats at T0, T2, and T7 of abstinence from ethanol in the insular cortex, peri/entorhinal cortex and piriform/apirform cortices. Scale bar = 50μm; *p < 0.05.
Vimentin Immunoreactivity Was Dramatically Increased Throughout the Hippocampus in Ethanol-Treated Rats
Following a detailed survey of vimentin+IR across cortical and limbic regions, the hippocampus and six additional regions (below) where immunoreactivity appeared visually distinct were chosen for further analysis. Vimentin immunoreactivity (vimentin+IR) was apparent in multiple hippocampal regions, with the highest average expression at the T7 time point. Five subregions of the hippocampus were analyzed separately as shown in Figure 4C. Vimentin+IR was significantly elevated above controls in the CA1 band (Figure 4A; F3,50=247.9, p<0.0001) at T2 (p<0.0001), T7 (p<0.0001), and T14 (p<0.0001); the CA2/CA3 bands (F3,50=112.4, p<0.0001) at T2 (p<0.0001), T7 (p<0.0001), and T14 (p<0.0001); the molecular layer (F3,50=141.8, p<0.0001) at T2 (p<0.0001), T7 (p<0.0001), and T14 (p<0.0001); and the hilus (F3,50=32.46, p<0.0001) at T2 (p<0.0001), T7 (p<0.0001), and T14 (p<0.0001). Vimentin+IR was largely absent from controls except for one region: the granule cell layer of the dentate gyrus. As a result, in this region controls were not collapsed, and are included in the analysis and graph (Figure 4B). Two-way ANOVA found a significant effect of ethanol treatment (F1,48=12.44, p=0.0009) and time-point (F2,48=9.601, p=0.0003), with Bonferroni’s multiple comparisons indicating a statistically significant increase in vimentin+IR of ethanol-treated animals over controls at the T14 time point (p=0.0203).
Figure 4:
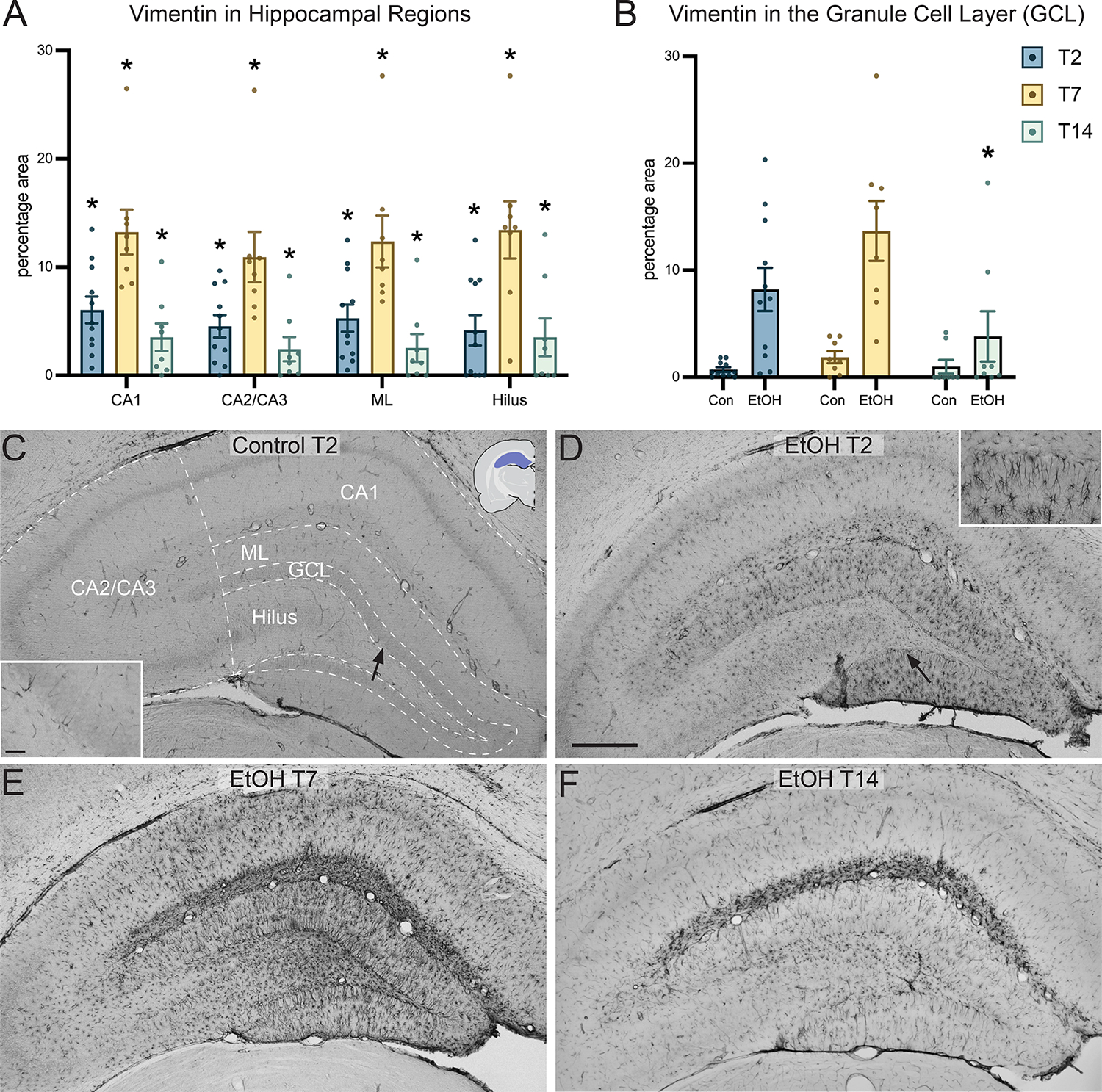
Vimentin immunoreactivity in the hippocampus. (A) Quantification of vimentin immunoreactivity in different hippocampal regions, the CA1 band, CA2/CA3 bands, molecular layer (ML) and the hilus at the T2, T7, and T14 time points. (B) Due to higher baseline immunoreactivity of neural stem/progenitor cells in the granule cell layer of the hippocampus, vimentin immunoreactivity was measured separately at the T2, T7, and T14 time points. (C) Representative 4x image of a control treated rat hippocampus at the T2 time point with hippocampal subregions delineated. (D-F) Representative images of the hippocampus of ethanol treated rats at T2, T7, and T14 time points. Both inserts are taken in the dentate gyrus, location is indicated by an arrow. Scale bar = 500 μm; insert scale bar = 50 μm; * p<0.05.
Ethanol Treatment Resulted in Increased Vimentin+IR in Cortical and Limbic Regions
Outside of the hippocampus, vimentin+IR appeared distinct in the somatosensory cortex, insular cortex, piriform/apiriform cortex, basolateral amygdala, peri/entorhinal cortex, and auditory cortex. Each region was found to have statistically significant elevated vimentin+IR over controls in at least one of the three time points (Figure 5A). Vimentin+IR was significantly elevated at the following time points in each brain region: the auditory cortex (F3,50=20.49, p<0.0001) at T2 (p=0.0001) and T7 (p<0.0001); the basolateral amygdala (F3,50=26.22, p<0.0001) at T2 (p=0.0001) and T7 (p<0.0001); the insular cortex (F3,50=20.36, p<0.0001) at T2 (p=0.0002) and T7 (p<0.0001); the peri/entorhinal cortex (F3,50=19.74, p<0.0001) at T2 (p<0.0001) and T7 (p<0.0001); the piriform/apiriform cortex (F3,50=22.94, p<0.0001) at T2 (p<0.0001) and T7 (p<0.0001); and the somatosensory cortex (F3,50=17.60, p<0.0001) at T2 (p=0.0030) and at T7 (p<0.0001).
Figure 5:
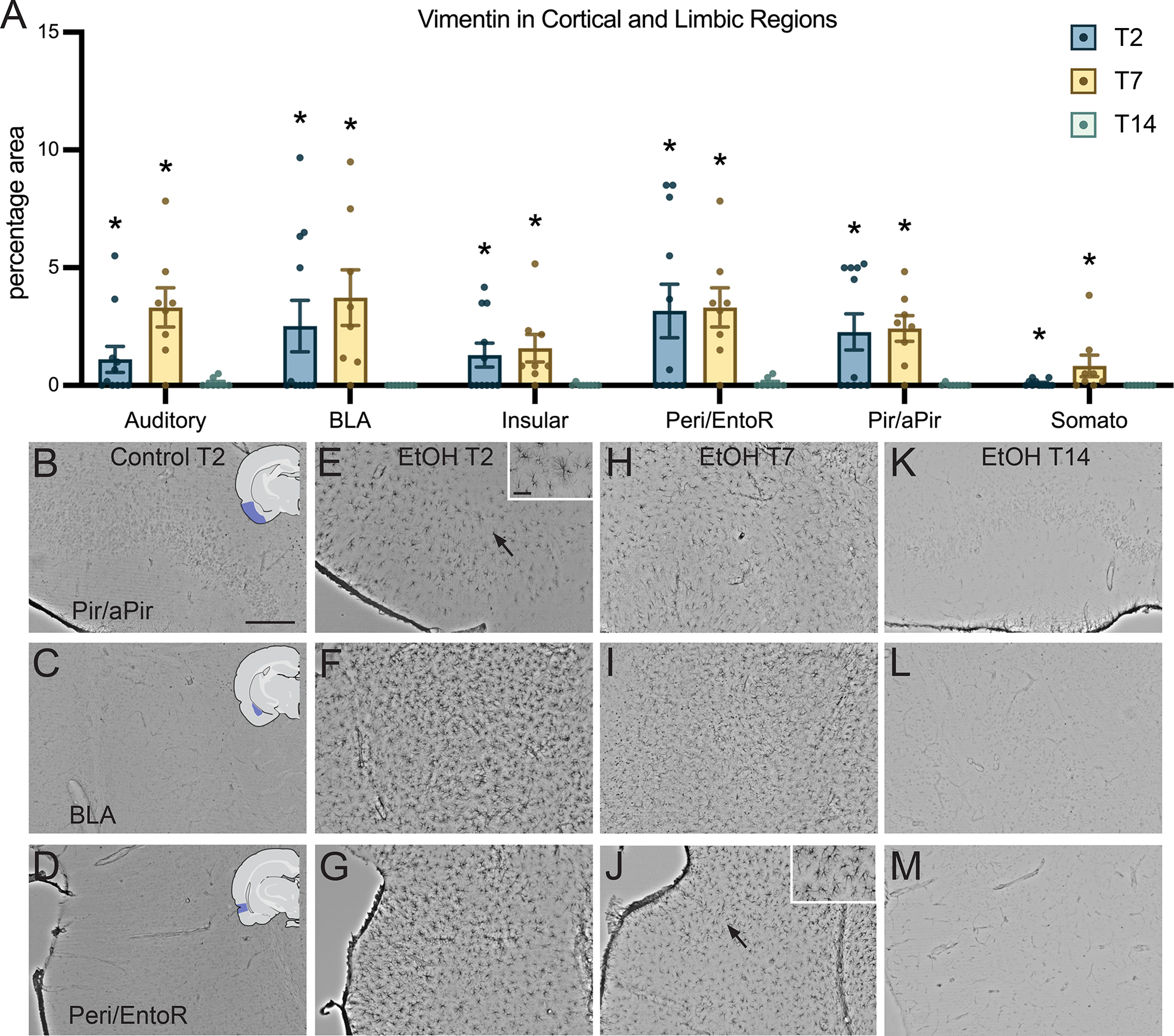
Vimentin immunoreactivity in cortical regions. (A) Quantification of vimentin immunoreactivity at T2, T7, and T14 in cortical regions: auditory cortex, the basolateral amygdala (BLA), the insular cortex, the peri/entorhinal cortex (Peri/EntoR), the piriform/apiriform cortex (Pir/aPir), and the somatosensory cortex (Somato). (B-M) Representative images of vimentin in control treated rats at T2, and ethanol treated rats at T2, T7 and T14 in the piriform/apiriform cortex, basolateral amygdala, and peri/entorhinal cortex. Scale bar = 250 μm; insert scale bar= 50 μm; * p<0.05.
Vimentin+ Cells are Astrocytes
To verify that vimentin+ cells were astrocytes, multiple fluorescent immunohistochemistry was carried out for vimentin plus GFAP (a marker for mature astrocytes) and vimentin plus Iba-1, a marker for microglia (Ahmed et al., 2007). When examined by eye, vimentin appeared to co-label with GFAP (Figure 6A–C, E), but not Iba-1 (Figure 6D), throughout the brain. This is consistent with the large majority of the recent literature (Potokar et al., 2020) despite earlier findings of vimentin present in microglia (Graeber et al., 1988). The vimentin signal also appeared to be stronger in astrocytes in the hippocampus than in the entorhinal cortex or the basolateral amygdala. Therefore, the coloc tool on Imaris software was employed to examine the colocalization of vimentin and GFAP across these regions. One-way ANOVA of Manders Correlation Coefficients indicated that the fraction of GFAP that colocalized with vimentin was indeed different among the three brain regions analyzed (F2,21=24.39, p<0.0001). Tukey’s multiple comparisons test revealed that colocalization of GFAP with vimentin was higher in the hippocampus compared to the entorhinal cortex (p<0.0001) or BLA (p<0.0001; Figure 6F).
Figure 6:
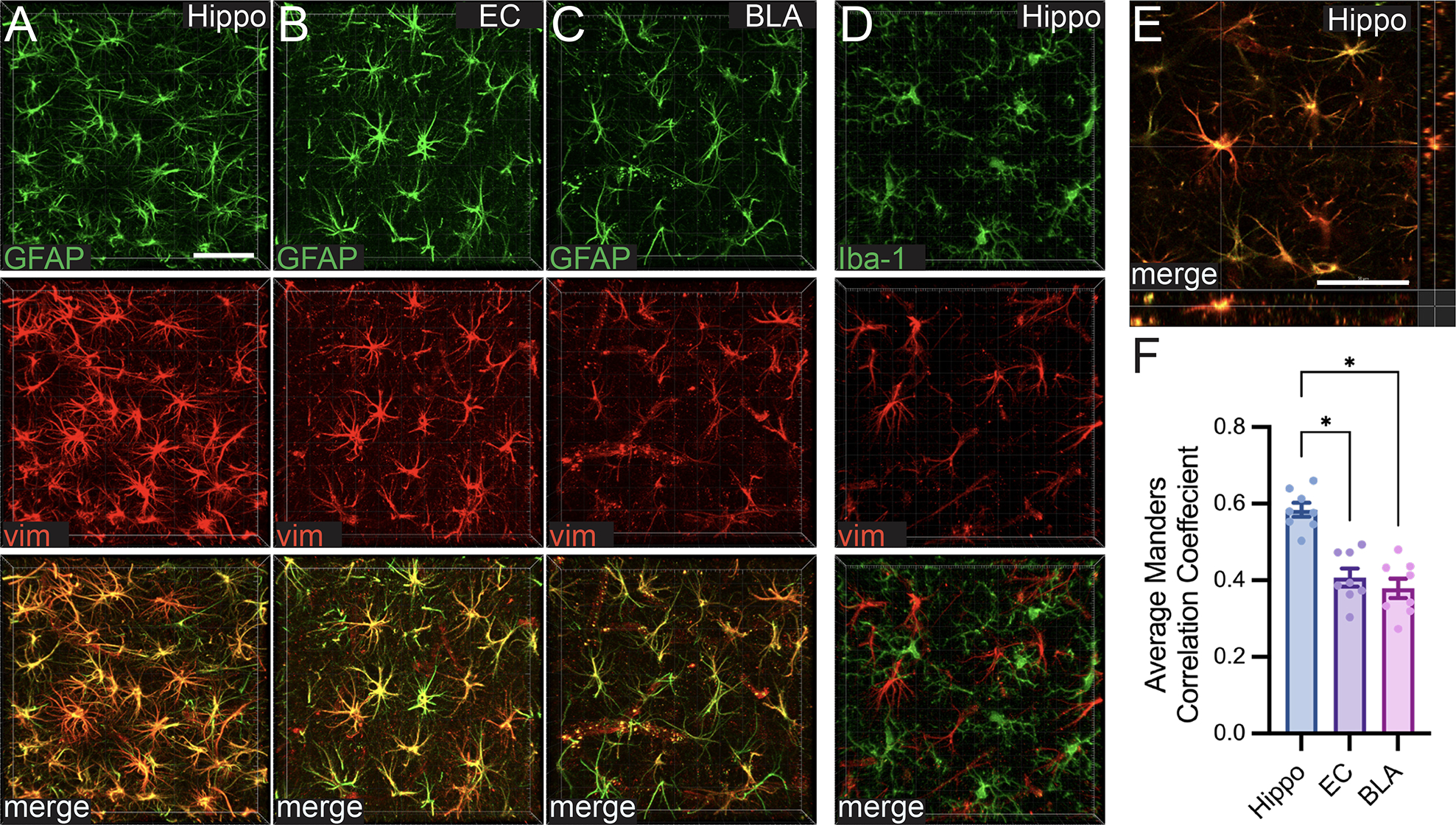
Z-stack images of vimentin colabeled with markers for astrocytes (A-C) but not microglia (D). (A-C) Representative images of a GFAP (green)/vimentin (red) colabel (merge image on the bottom) taken in the hippocampus (Hippo), entorhinal cortex (EC), and basolateral amygdala (BLA) of an ethanol treated rat at T7. (D) Representative 3D rendered z-stack images of an Iba-1 (green)/vimentin (red) colabel (merge image on the bottom) taken in the hippocampus of an ethanol treated rat at T7. (E) Orthogonal view of the merged GFAP/vimentin colabel in B to show vimentin and GFAP labeling the same cell. (F) The overlap of GFAP with vimentin quantified using the average Manders Correlation Coefficient in the three brain regions examined. Both scale bars= 50μm; * p<0.05.
Discussion
While animal models have been used to great effect to elucidate specific regions and time courses of alcohol-related neurodegeneration, most studies use only males. Therefore, even if both males and females are used, as is slowly becoming the expectation, the regions and time points chosen for analysis are often biased by previous studies which omitted females. Given the context of increasing alcohol use among women, the need to understand how alcohol may uniquely affect women versus men is long overdue (White, 2020). Therefore, this study sought to focus only on female rats and characterize, in detail, the presence of two markers of alcohol-induced damage throughout the brain at multiple time points. To do so, FJB staining, a reliable and highly sensitive method of labeling degenerating neurons (Schmued & Hopkins, 2000) was quantified throughout cortical and limbic regions. The brains of female rats exposed to ethanol contained FJB+ cells in multiple cortical and limbic regions previously unreported in males. Next, vimentin, an intermediate filament protein upregulated in reactive glial cells in several neurodegenerative diseases (Chen et al., 2022; Yamada et al., 1992) was examined as another metric of potential damage. Vimentin+ cells co-labeled with the astrocyte marker GFAP (Figure 6) and did not share any overlap with microglia marker, Iba-1, suggesting that these cells were indeed reactive astrocytes. Additionally, vimentin+ reactive astrocytes appeared at an earlier time point in ethanol-exposed females compared to what has been observed in males (see Figure 7 for a summary of regions where FJB and vimentin+ cells were observed, yellow highlights indicate regions that may be unique to females). Thus, these findings suggest that alcohol induced neurodegeneration could occur earlier and in different regions compared to males. These data have important implications for our understanding of how alcohol affects the female brain and ultimately how these changes underlie the development of AUDs.
Figure 7:
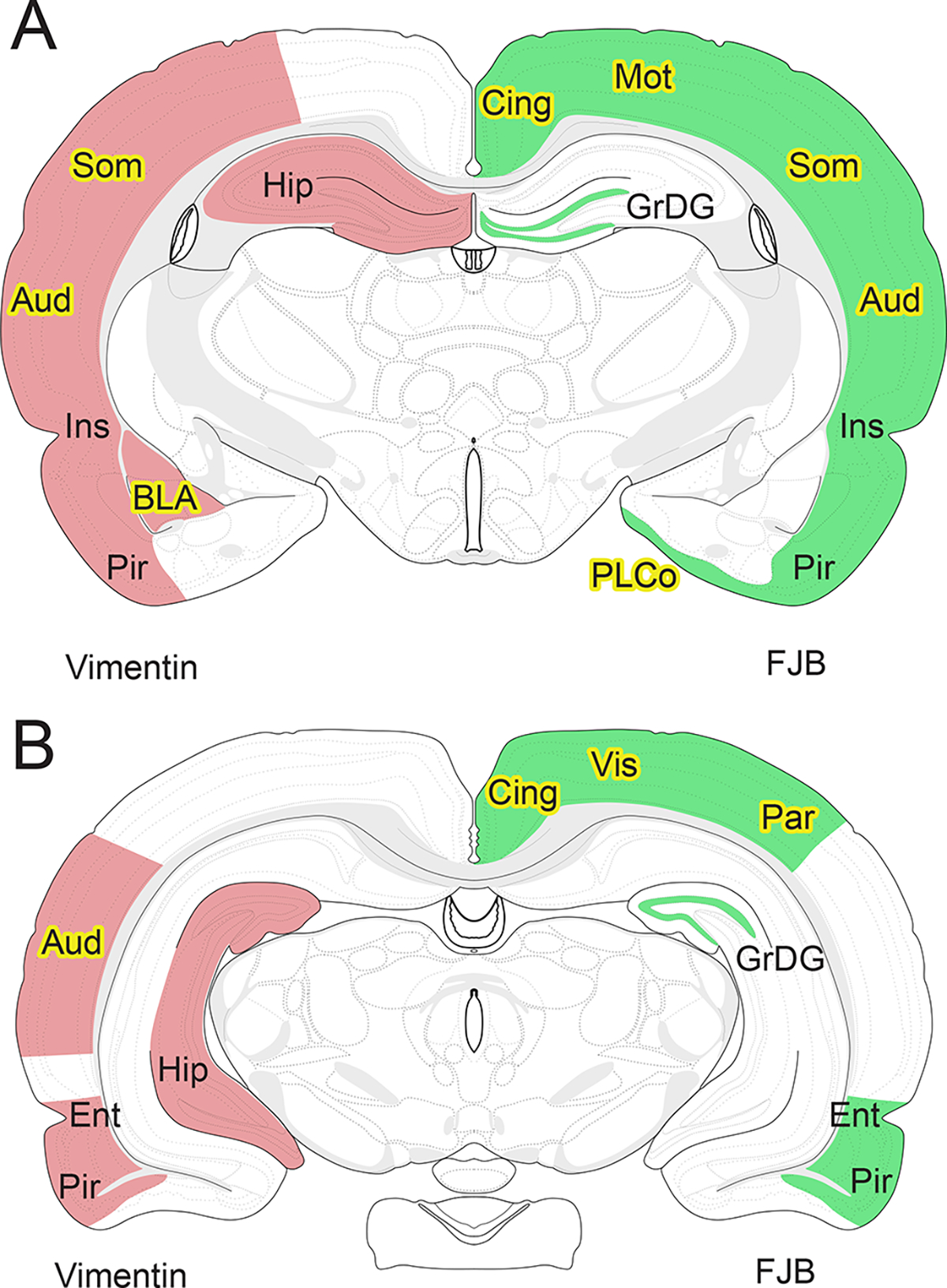
A comparison of brain regions damaged by alcohol in female versus male rats after alcohol dependence in a 4-day binge model. Regions with statistically significant increases in vimentin (pink) or FluoroJade-B (green) over controls in ethanol treated female rats. Yellow highlights indicate regions where statistically significant vimentin staining or FluoroJade-B was not previously reported in males. (A) Coronal view of the rat brain at −3.00mm Bregma and at (B) −5.00mm Bregma. Label abbreviations are as follows. GrDG: granule cell layer of the dentate gyrus, Ins: insular cortex, Pir: piriform/apiriform cortex, BLA: basolateral amygdala, Hip: hippocampus, Cing: cingulate/retrosplenial cortex, Mot: motor cortex, Som: somatosensory cortex, Aud: auditory cortex, PLCo: posterolateral cortical amygdaloid nucleus, Vis: visual cortex, Par: parietal cortex, Ent: peri/entorhinal cortex. Comparison is to male data from: Collins et al., 1996; Crews et al., 2000; Hayes et al., 2013; Kelso et al., 2011; Obernier, Bouldin, et al., 2002).
A thorough survey throughout the brain was undertaken to identify possible regional differences in FJB staining following alcohol administration. Although this approach required extensive effort, it eliminated the bias of only looking at regions where damage had been reported in males. The FJB method for detecting neuronal degeneration compares favorably with more traditional methods such as silver stain (Anderson et al., 2005; Kelso et al., 2011) but appears to label a later phase of cell death (Poirier et al., 2000). FJB+ cells were significantly elevated in the same brain regions previously reported in males (Corso et al., 1998; Crews et al., 2000; Hayes et al., 2013; Kelso et al., 2011; Obernier, Bouldin, et al., 2002; Walker et al., 1980) including the insular cortex, piriform cortex, peri/entorhinal cortex, and the dentate gyrus granule cell layer. Several new regions of degeneration emerged: the posterolateral cortical amygdaloid nucleus and the cingulate/retrosplenial, motor, parietal, somatosensory, and visual cortices, though reactive microglia - which suggested some level of perturbation - were observed by us in some of these regions in males (Nixon et al., 2008). Like in males (Kelso et al., 2011), and similar to other indicators of degeneration such as amino cupric silver stain (Obernier, White, et al., 2002), FJB+ cells were highest in most brain regions immediately following the end of binge ethanol administration, with degeneration continuing at lower but still detectable levels during abstinence from ethanol at T2 and T7. At T14 almost no FJB+ cells were found in any brain regions. Though the number of FJB+ cells appears to fluctuate across time points (e.g., Figure 3), T2 does not differ significantly from T0 or T7. Furthermore, any perceived difference may not be meaningful given the high variability in cell death markers historically noted in this model, though this point is only based on observations from male rats (see Crews et al., 2000; Corso et al., 1998; Collins et al..1996 and especially Kelso et al., 2011). Cell death observed at the T2 or T7 time points theoretically could be a response to reactive microglia rather than alcohol itself (Crews & Nixon, 2009). However, our recent work examining microglia in female rats is highly similar to our past work in males (Morris, Kelso, et al., 2010; Peng et al., 2017): a modest microglial reaction occurs, based on Iba1+ cell morphology and cytokine expression in hippocampal and entorhinal cortex homogenates (Melbourne et al., in preparation). Furthermore, previous reports in males have identified the method of cell death in this model as necrotic through careful ultrastructural examination, and a lack of TUNEL staining, an indicator of apoptosis (Morris, Eaves, et al., 2010; Obernier, Bouldin, et al., 2002). A lack of TUNEL staining has been confirmed with female rats as well (Maynard et al., 2018). Therefore, while cell death appears in female rats at similar time points as in males following binge ethanol exposure, there appear to be some regional differences.
Additional regions of cell death in females compared to males could underlie certain vulnerabilities to AUD in women. These previously unreported areas containing FJB+ cells were widespread in corticolimbic regions in female rats. Although some studies fail to find any sex differences when comparing the brain volumes of men and women with AUD (Demirakca et al., 2011; Mechtcheriakov et al., 2007), those that do typically note differences in the cortex, particularly in cortical thickness (Momenan et al., 2012; Thayer et al., 2016). As shown in this study (Figure 3), disparities in cortical thickness could be explained by cell death in more cortical sub-regions in females compared to males. Furthermore, some of the cortical subregions observed in this study to contain FJB+ cells may also be involved in the progression and outcomes of AUD. For instance, the cingulate cortex is involved in reward-based decision-making (Bush et al., 2002), and size reductions in this region are observed in an animal model of AUD (Zhao et al., 2021). In those with AUD, reduced cingulate connectivity occurs, and serves as a marker of future relapse (Zakiniaeiz et al., 2017). Deficits in the parietal cortex may be responsible for decreased performance in visuospatial tasks in both men and women with AUD (Sullivan et al., 2000, 2002). And impairments in visual learning commonly observed in those with AUD (Stavro et al., 2013) may relate to neuronal cell loss or altered cellular metabolism in the visual cortex (Bagga et al., 2014). The presence of cell death in these additional brain regions in females may therefore facilitate the development of AUD and/or impact maintaining abstinence and recovery.
Vimentin reactivity in this study with females was more regionally and temporally widespread compared to previous reports using male rats. Vimentin immunoreactivity in female rats was noted in all brain regions previously reported in males (piriform/apiriform cortex, peri/entorhinal cortex, insular cortex, and hippocampus), and three previously unreported regions: somatosensory cortex, basolateral amygdala, and auditory cortex (Figure 5). Vimentin immunoreactivity was highest at T7, similar to peak reactivity in males (Kelso et al., 2011). However, most cortical regions and all hippocampal regions had significantly increased vimentin expression after only two days of abstinence following ethanol exposure compared to four days in males. Additionally, in all hippocampal regions, the timeline of immunoreactivity persisted compared to males, with significant vimentin immunoreactivity at the T14 time point. These findings complement other reports examining sex differences in astrocyte reactivity following ethanol exposure. In adult mice after chronic ethanol exposure, GFAP density is increased in the hippocampus of females but not males (Wilhelm et al., 2016). These authors also noted increased Tnf mRNA expression in astrocyte cultures from females but not males following ethanol exposure, which suggests that these astrocytes may be of a more pro-inflammatory phenotype. In contrast, more modest exposure in adolescent rats increased GFAP immunoreactivity following 30 days abstinence in the hippocampus of both females and males (Nwachukwu et al., 2022). Sex differences were suggested as there were fewer GFAP+ cells in the CA2/3 and dentate gyrus of females, although these studies did not use stereology and the reduced number of cells may simply reflect that females are physically smaller than males. Similarly, in a mouse model of chronic alcohol exposure decreased GFAP expression occurred in the hippocampus of females but not males, although only the dentate gyrus was examined (McGrath et al., 2017). Further evidence for sex differences is observed in other brain regions. Ethanol exposure blunts GFAP immunoreactivity in male mice, but not females in the BLA (Brewton et al., 2023). Gene expression is altered in a sexually dimorphic manner in response to mouse ethanol exposure, with skewed enrichment of reactive astrocyte genes in the medial prefrontal cortex of females (Wilhelm et al., 2015). In ethanol dependent mice, reductions in GFAP immunoreactivity are found the nucleus accumbens, suggesting altered astrocyte function (Giacometti et al., 2020). Overall, these differences suggest that vimentin+IR and therefore astrocyte reactivity may occur differently in females compared to males following ethanol administration, although effects are variable and may be influenced by the animal model of alcohol use, time points chosen, and the regions examined.
The dramatic upregulation of vimentin observed in female rats may be important for helping astrocytes restore brain homeostasis altered during alcohol use. Normally, vimentin is not expressed by many astrocytes in the adult brain, and usually only appears in astrocytes before cortical myelination (Dahl, 1981; Sancho-Tello et al., 1995) or in radial glial stem cells (Schnitzer et al., 1981). Only under pathological conditions like heavy alcohol use is vimentin expressed at high levels in mature astrocytes. Vimentin is required for the formation of other intermediate filaments like synemin (Jing et al., 2007), and possibly GFAP as well (Galou et al., 1996). These intermediate filaments, including vimentin, are involved in several processes which may help reactive astrocytes respond to their environment. Upregulation of intermediate filaments aids in vesicular mobility, potentially allowing astrocytes to more efficiently release factors in response to the acute stages of injury (Potokar et al., 2010). Intermediate filament upregulation also allows astrocytes to more efficiently deliver MHC class II molecules to the cell surface, which may allow astrocytes to better respond to neuroimmune insults (Vardjan et al., 2012). In line with these roles, glial scar formation following brain lesion (Pekny et al., 1999) and neurological recovery following stroke (Liu et al., 2014) are both impaired when intermediate filaments are knocked out in animal models. Interestingly, when the degree of colocalization of GFAP with vimentin was quantified in several brain regions, GFAP positive astrocytes correlated more strongly with vimentin-labeled cells in the hilus of the hippocampus (an area in the vicinity of both robust cell death, and the neurogenic dentate gyrus granule cell layer) than in the entorhinal cortex or basolateral amygdala (Figure 6). This area of adult neurogenesis is visible in control images of the dentate gyrus (see insert, Figure 4) as quiescent neural progenitor cells label positive for vimentin (Encinas et al., 2006). Upregulation of vimentin may therefore be a mechanism for reactive astrocytes to respond to an altered environment quickly and effectively.
Gonadal hormones could contribute to some of the potential sex differences in vimentin immunoreactivity exhibited by the female rats in this study. Astrocytes express both estrogen receptor beta and alpha (Azcoitia et al., 1999; Pawlak et al., 2005). They are also highly steroidogenic and capable of producing progesterone, testosterone, and estradiol (Zwain & Yen, 1999). Consequently, estrogen can impact multiple measures of astrocyte reactivity. In studies of animal models of ischemia, estrogen treatment reduces both astrocyte proliferation and number in the vicinity of the injury (Garcia-Estrada et al., 1993; García-Estrada et al., 1999). However, GFAP immunoreactivity can vary based on the estrous cycle (Arias et al., 2009), with ischemia-induced increases in GFAP greater in female mice in the diestrus period compared to males (Cordeau et al., 2008). In this study, vimentin immunoreactivity varied substantially among subjects, with some ethanol-treated rats exhibiting very high amounts of vimentin expression and other rats very little. We did not assess for the estrous cycle in these rats, so the variability in stages of the estrous cycle across rats theoretically could have contributed to this differential expression of vimentin. However, there is growing evidence that the estrous cycle contributes little to variability of behavior in rodents (Dayton et al., 2016; Levy et al., 2023), though it is well known that estrogen has anti-inflammatory and neuroprotective roles in neurodegenerative conditions (Crespo-Castrillo & Arevalo, 2020). Furthermore, variability in vimentin+IR was also very high among males subjects (Hayes et al., 2013; Kelso et al., 2011), so the effects of estrogen may be modest. To fully resolve this question, it might be of interest to measure the estrous cycle in future studies.
Astrocyte reactivity has significant functional implications (Escartin et al., 2019). In many neurodegenerative conditions, astrocyte homeostatic functions such as neurotransmitter and ion buffering, gliotransmitter release, cytokine, and growth factor release, phagocytosis, and production or detoxification of reactive oxygen species are altered (Escartin et al., 2019, 2021). These changes may be either enhanced or reduced, and importantly are disease/insult-specific (Escartin et al., 2019). While some groups have examined the role of astrocytes or astrocytic glutamate transporters in addiction behaviors (Alhaddad et al., 2022; Bull et al., 2014; Nwachukwu et al., 2021; Rao et al., 2015), how astrocytes alter glutamate homeostasis following alcohol dependence is only beginning to be examined (Adermark & Bowers, 2016; Miguel-Hidalgo, 2021). In ethanol preferring rats, increased glutamine synthetase-positive astrocytes are observed after chronic alcohol drinking (Miguel-Hidalgo, 2006), which may contribute to the known glutamate dysregulation that occurs with alcohol dependence (Adermark & Bowers, 2016). Thus, reactive astrocytes are likely redirected from homeostatic activities like glutamate uptake, potentially altering glutamatergic transmission and synaptic plasticity (Ayers-Ringler et al., 2016; Mulholland et al., 2009; Smith, 1997).
An intriguing story is emerging on the large functional diversity in astrocyte reactivity (Liddelow & Barres, 2017). Our assessment of key cytokines and growth factors supports a more anti-inflammatory or pro-regeneration environment in both males (Peng et al., 2017; Peng & Nixon, 2020) and females (Melbourne et al., in prep). Work in rats, however, is in direct contrast to recent work in C57Bl/6J mice that find a transcriptomic signature of an acute injury phenotype (Holloway et al., 2023). C57Bl/6J mice have a mutation in the nnt gene that renders them more susceptible to oxidative stress and inflammation. Indeed, this mutation may explain why C57Bl/6J mice consistently have pro-inflammatory effects to ethanol (Kane & Drew, 2016; Qin et al., 2008), while most rats studies do not (Bell-Temin et al., 2013; Gano et al., 2016; Marshall et al., 2013; Peng et al., 2017; Peng & Nixon, 2020; Zahr et al., 2010). The possibility that reactive astrocytes after alcohol dependence may have a beneficial phenotype is supported by the absence of significant astrocyte proliferation (Adermark & Bowers, 2016; Nixon & Crews, 2004) or scarring, both of which occur in conditions with more significant cell death events such as TBI or ischemia (Burda et al., 2016; Pekny & Nilsson, 2005)
This study sought to fill in an important gap in the literature and examine how excessive alcohol exposure may uniquely affect the female brain by examining multiple brain regions at several time points following ethanol exposure. A direct comparison of males and females was not made in the interest of reducing the use of animals by not duplicating past work, as these studies could be conducted identically to past work in males with overlapping personnel. Thus, when comparing to past work by ourselves and others (Crews et al., 2000; Kelso et al., 2011; Walker et al., 1980) these data suggest that alcohol induced neurodegeneration both earlier and later and in different regions compared to males. Neuronal degeneration appeared in additional brain regions compared to past studies with males. In female rats, vimentin+ astrocytes appeared at an earlier and later time point, and in some previously undescribed regions. This may indicate that the female brain is more vulnerable to alcohol-induced degeneration. This study provides a strong foundation to better understand the potentially sex-specific mechanisms that underlie alcohol-induced brain damage in AUDs. Understanding how alcohol use affects men and women differently will be key to designing better more targeted therapeutic strategies for AUDs.
Supplementary Material
Supplementary Figure 1: Additional images of FJB stained tissue not shown in Figure 2. Controls for each time point and an additional region, the posterolateral cortical amygdaloid nucleus (PLCo) are shown. Representative images are taken at the T0, T2, T7 and T14 time points. Scale bar = 50μm.
Supplementary Figure 2: Additional images of vimentin immunoreactivity in cortical regions not shown in Figure 5. Controls for each time point and an additional region, the auditory cortex, are shown. Representative images are taken at the T2, T7 and T14 time points. Scale bar = 250 μm.
Funding
This research was funded by NIH grants R01AA016959 (KN), R01AA016959–11S1 (KN/SPG), T32AA007471 (SPG), and 1F31AA030720–01 (SPG), and start-up funds from The University of Texas at Austin College of Pharmacy.
Footnotes
Competing Interests
Declarations of interest: none
Ethics Approval and Consent to Participate
The animal study was reviewed and approved by the University of Kentucky Institutional Animal Care and Use Committee and The University of Texas at Austin Institutional Animal Care and Use Committee.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Bibliography
- Acker C (1985). Performance of female alcoholics on neuropsychological testing. Alcohol and Alcoholism (Oxford, Oxfordshire), 20(4), 379–386. [PubMed] [Google Scholar]
- Acker C (1986). Neuropsychological deficits in alcoholics: The relative contributions of gender and drinking history. British Journal of Addiction, 81(3), 395–403. 10.1111/j.1360-0443.1986.tb00346.x [DOI] [PubMed] [Google Scholar]
- Adermark L, & Bowers MS (2016). Disentangling the Role of Astrocytes in Alcohol Use Disorder. Alcoholism: Clinical and Experimental Research, 40(9), 1802–1816. 10.1111/acer.13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, & Hommer DW (1999). Hippocampal Volume in Patients With Alcohol Dependence. Archives of General Psychiatry, 56(4), 356–363. 10.1001/archpsyc.56.4.356 [DOI] [PubMed] [Google Scholar]
- Agartz I, Shoaf S, Rawlings RR, Momenan R, & Hommer DW (2003). CSF monoamine metabolites and MRI brain volumes in alcohol dependence. Psychiatry Research, 122(1), 21–35. 10.1016/s0925-4927(02)00084-7 [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, & Dickson DW (2007). Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society, 55(7), 687–700. 10.1369/jhc.6A7156.2007 [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Wong W, Abou-Gharbia M, Childers W, Melenski E, Bell RL, & Sari Y (2022). Effects of a Novel Beta Lactam Compound, MC-100093, on the Expression of Glutamate Transporters/Receptors and Ethanol Drinking Behavior of Alcohol-Preferring Rats. Journal of Pharmacology and Experimental Therapeutics, 383(3), 208–216. 10.1124/jpet.122.001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KJ, Miller KM, Fugaccia I, & Scheff SW (2005). Regional distribution of Fluoro-Jade B staining in the hippocampus following traumatic brain injury. Experimental Neurology, 193(1), 125–130. 10.1016/j.expneurol.2004.11.025 [DOI] [PubMed] [Google Scholar]
- Arias C, Zepeda A, Hernández-Ortega K, Leal-Galicia P, Lojero C, & Camacho-Arroyo I (2009). Sex and estrous cycle-dependent differences in glial fibrillary acidic protein immunoreactivity in the adult rat hippocampus. Hormones and Behavior, 55(1), 257–263. 10.1016/j.yhbeh.2008.10.016 [DOI] [PubMed] [Google Scholar]
- Ashley MJ, Olin JS, le Riche WH, Kornaczewski A, Schmidt W, & Rankin JG (1977). Morbidity in alcoholics. Evidence for accelerated development of physical disease in women. Archives of Internal Medicine, 137(7), 883–887. 10.1001/archinte.137.7.883 [DOI] [PubMed] [Google Scholar]
- Ayers-Ringler JR, Jia Y-F, Qiu Y-Y, & Choi D-S (2016). Role of astrocytic glutamate transporter in alcohol use disorder. World Journal of Psychiatry, 6(1), 31–42. 10.5498/wjp.v6.i1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, & Garcia-Segura LM (1999). Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia, 26(3), 260–267. [PubMed] [Google Scholar]
- Bagga D, Khushu S, Modi S, Kaur P, Bhattacharya D, Garg ML, & Singh N (2014). Impaired visual information processing in alcohol-dependent subjects: A proton magnetic resonance spectroscopy study of the primary visual cortex. Journal of Studies on Alcohol and Drugs, 75(5), 817–826. 10.15288/jsad.2014.75.817 [DOI] [PubMed] [Google Scholar]
- Baldwin SA, & Scheff SW (1996). Intermediate filament change in astrocytes following mild cortical contusion. Glia, 16(3), 266–275. [DOI] [PubMed] [Google Scholar]
- Barton EA, Baker C, & Leasure JL (2017). Investigation of Sex Differences in the Microglial Response to Binge Ethanol and Exercise. Brain Sciences, 7(10), Article 10. 10.3390/brainsci7100139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Temin H, Zhang P, Chaput D, King MA, You M, Liu B, & Stevens SM (2013). Quantitative proteomic characterization of ethanol-responsive pathways in rat microglial cells. Journal of Proteome Research, 12(5), 2067–2077. 10.1021/pr301038f [DOI] [PubMed] [Google Scholar]
- Blanco AM, Pascual M, Valles SL, & Guerri C (2004). Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport, 15(4), 681–685. 10.1097/00001756-200403220-00021 [DOI] [PubMed] [Google Scholar]
- Brewton HW, Robinson SL, & Thiele TE (2023). Astrocyte expression in the extended amygdala of C57BL/6J mice is sex-dependently affected by chronic intermittent and binge-like ethanol exposure. Alcohol, 108, 55–64. 10.1016/j.alcohol.2022.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, & Bowers MS (2014). Rat Nucleus Accumbens Core Astrocytes Modulate Reward and the Motivation to Self-Administer Ethanol after Abstinence. Neuropsychopharmacology, 39(12), Article 12. 10.1038/npp.2014.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, & Sofroniew MV (2016). Astrocyte roles in traumatic brain injury. Experimental Neurology, 275, 305–315. 10.1016/j.expneurol.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, & Rosen BR (2002). Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America, 99(1), 523–528. 10.1073/pnas.012470999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-Z, Liu S-X, Li Y-W, He T, Zhao J, Wang T, Qiu X-X, & Wu H-F (2022). Vimentin as a potential target for diverse nervous system diseases. Neural Regeneration Research, 18(5), 969–975. 10.4103/1673-5374.355744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H, & Lee CJ (2018). Reactive astrocytes in Alzheimer’s disease: A double-edged sword. Neuroscience Research, 126, 44–52. 10.1016/j.neures.2017.11.012 [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, & Neafsey EJ (1996). Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: Possible explanation for olfactory deficits in alcoholics. Alcoholism, Clinical and Experimental Research, 20(2), 284–292. 10.1111/j.1530-0277.1996.tb01641.x [DOI] [PubMed] [Google Scholar]
- Cordeau P, Lalancette-Hébert M, Weng YC, & Kriz J (2008). Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke, 39(3), 935–942. 10.1161/STROKEAHA.107.501460 [DOI] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, & Neafsey EJ (1998). Brain Neuronal Degeneration Caused by Episodic Alcohol Intoxication in Rats: Effects of Nimodipine, 6,7-Dinitro-quinoxaline-2,3-dione, and MK-801. Alcohol: Clinical and Experimental Research, 22(1), 217–224. 10.1111/j.1530-0277.1998.tb03641.x [DOI] [PubMed] [Google Scholar]
- Cortez I, Rodgers SP, Kosten TA, & Leasure JL (2020). Sex and Age Effects on Neurobehavioral Toxicity Induced by Binge Alcohol. Brain Plasticity, 6(1), 5–25. 10.3233/BPL-190094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Castrillo A, & Arevalo M-A (2020). Microglial and Astrocytic Function in Physiological and Pathological Conditions: Estrogenic Modulation. International Journal of Molecular Sciences, 21(9), Article 9. 10.3390/ijms21093219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, & Knapp DJ (2000). Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism, Clinical and Experimental Research, 24(11), 1712–1723. [PubMed] [Google Scholar]
- Crews FT, & Nixon K (2009). Mechanisms of Neurodegeneration and Regeneration in Alcoholism. Alcohol and Alcoholism, 44(2), 115–127. 10.1093/alcalc/agn079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D (1981). The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination. Journal of Neuroscience Research, 6(6), 741–748. 10.1002/jnr.490060608 [DOI] [PubMed] [Google Scholar]
- Dayton A, Exner EC, Bukowy JD, Stodola TJ, Kurth T, Skelton M, Greene AS, & Cowley AW (2016). Breaking the Cycle. Hypertension, 68(5), 1139–1144. 10.1161/HYPERTENSIONAHA.116.08207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kämmerer N, Welzel-Marquez H, Hermann D, Heinz A, & Mann K (2011). Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcoholism, Clinical and Experimental Research, 35(9), 1678–1685. 10.1111/j.1530-0277.2011.01514.x [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, & Martin PR (1986). Clinical assessment of cognition in alcoholism. Alcoholism: Clinical and Experimental Research, 10, 123–127. 10.1111/j.1530-0277.1986.tb05058.x [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, & Enikolopov G (2006). Fluoxetine targets early progenitor cells in the adult brain. Proceedings of the National Academy of Sciences of the United States of America, 103(21), 8233–8238. 10.1073/pnas.0601992103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen W-T, Cohen-Salmon M, … Verkhratsky A (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 1–14. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Guillemaud O, & Sauvage M-AC (2019). Questions and (some) answers on reactive astrocytes. Glia, 67(12), 2221–2247. 10.1002/glia.23687 [DOI] [PubMed] [Google Scholar]
- Fama R, Le Berre A-P, & Sullivan EV (2020). Alcohol’s Unique Effects on Cognition in Women: A 2020 (Re)view to Envision Future Research and Treatment. Alcohol Research: Current Reviews, 40(2), 03. 10.35946/arcr.v40.2.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H (1995). Influence of chronic alcohol treatment on the GFAP-immunoreactivity in astrocytes of the hippocampus in rats. Acta Histochemica, 97(3), 263–271. 10.1016/S0065-1281(11)80187-X [DOI] [PubMed] [Google Scholar]
- Galou M, Colucci-Guyon E, Ensergueix D, Ridet JL, Gimenez y Ribotta M, Privat A, Babinet C, & Dupouey P (1996). Disrupted glial fibrillary acidic protein network in astrocytes from vimentin knockout mice. The Journal of Cell Biology, 133(4), 853–863. 10.1083/jcb.133.4.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL, & Deak T (2016). Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Research, 1646, 62–72. 10.1016/j.brainres.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, & Garcia-Segura LM (1993). Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Research, 628(1–2), 271–278. 10.1016/0006-8993(93)90964-o [DOI] [PubMed] [Google Scholar]
- García-Estrada J, Luquín S, Fernández AM, & Garcia-Segura LM (1999). Dehydroepiandrosterone, pregnenolone and sex steroids down-regulate reactive astroglia in the male rat brain after a penetrating brain injury. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 17(2), 145–151. 10.1016/s0736-5748(98)00065-3 [DOI] [PubMed] [Google Scholar]
- Paxinos George & Watson Charles. (2009). The Rat Brain in Stereotaxic Coordinates (Compact 6th). Elsevier Academic. [Google Scholar]
- Giacometti LL, Chandran K, Figueroa LA, & Barker JM (2020). Astrocyte modulation of extinction impairments in ethanol-dependent female mice. Neuropharmacology, 179, 108272. 10.1016/j.neuropharm.2020.108272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni F, & Quintana FJ (2020). The Role of Astrocytes in CNS Inflammation. Trends in Immunology, 41(9), 805–819. 10.1016/j.it.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, & Kreutzberg GW (1988). The microglial cytoskeleton: Vimentin is localized within activated cellsin situ. Journal of Neurocytology, 17(4), 573–580. 10.1007/BF01189811 [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, & Hasin DS (2017). Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(9), 911–923. 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, & Hasin DS (2015). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72(8), 757–766. 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Sher KJ, Kerr WC, Krauss MJ, Lui CK, McDowell YE, Hartz S, Virdi G, & Bierut LJ (2018). Trends in Adult Alcohol Use and Binge Drinking in the Early 21st-Century United States: A Meta-Analysis of 6 National Survey Series. Alcoholism, Clinical and Experimental Research, 42(10), 1939–1950. 10.1111/acer.13859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DM, Deeny MA, Shaner CA, & Nixon K (2013). Determining the Threshold for Alcohol-Induced Brain Damage: New Evidence with Gliosis Markers. Alcoholism: Clinical and Experimental Research, 37(3), 425–434. 10.1111/j.1530-0277.2012.01955.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway KN, Pinson MR, Douglas JC, Rafferty TM, Kane CJM, Miranda RR, & Drew PD (2023). Cerebellar Transcriptomic Analysis in a Chronic plus Binge Mouse Model of Alcohol Use Disorder Demonstrates Ethanol-Induced Neuroinflammation and Altered Glial Gene Expression. Cells, 12(5), Article 5. 10.3390/cells12050745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, & Rawlings R (2001). Evidence for a gender-related effect of alcoholism on brain volumes. The American Journal of Psychiatry, 158(2), 198–204. 10.1176/appi.ajp.158.2.198 [DOI] [PubMed] [Google Scholar]
- Jing R, Wilhelmsson U, Goodwill W, Li L, Pan Y, Pekny M, & Skalli O (2007). Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. Journal of Cell Science, 120(Pt 7), 1267–1277. 10.1242/jcs.03423 [DOI] [PubMed] [Google Scholar]
- Kane CJM, & Drew PD (2016). Inflammatory responses to alcohol in the CNS: Nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. Journal of Leukocyte Biology, 100(5), 951–959. 10.1189/jlb.3MR0416-171R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, & Nixon K (2011). Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience, 197, 381–393. 10.1016/j.neuroscience.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, & Hasin DS (2008). Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug and Alcohol Dependence, 93(1–2), 21–29. 10.1016/j.drugalcdep.2007.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Li G, & Hasin DS (2011). Birth cohort effects and gender differences in alcohol epidemiology: A review and synthesis. Alcoholism, Clinical and Experimental Research, 35(12), 2101–2112. 10.1111/j.1530-0277.2011.01562.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, & Cartwright H (1997). The cerebral cortex is damaged in chronic alcoholics. Neuroscience, 79(4), 983–998. 10.1016/S0306-4522(97)00083-3 [DOI] [PubMed] [Google Scholar]
- Leasure JL, & Nixon K (2010). Exercise Neuroprotection in a Rat Model of Binge Alcohol Consumption. Alcoholism, Clinical and Experimental Research, 34(3), 404–414. 10.1111/j.1530-0277.2009.01105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DR, Hunter N, Lin S, Robinson EM, Gillis W, Conlin EB, Anyoha R, Shansky RM, & Datta SR (2023). Mouse spontaneous behavior reflects individual variation rather than estrous state. Current Biology: CB, S0960–9822(23)00175–6. 10.1016/j.cub.2023.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Ståhlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, … Pekny M (2008). Protective role of reactive astrocytes in brain ischemia. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 28(3), 468–481. 10.1038/sj.jcbfm.9600546 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, & Barres BA (2017). Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity, 46(6), 957–967. 10.1016/j.immuni.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, … Barres BA (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), Article 7638. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, & Chopp M (2014). Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia, 62(12), 2022–2033. 10.1002/glia.22723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E (1975). Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia, 43(3), 245–254. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, & Diehl A (2005). Neuroimaging of gender differences in alcohol dependence: Are women more vulnerable? Alcoholism, Clinical and Experimental Research, 29(5), 896–901. 10.1097/01.alc.0000164376.69978.6b [DOI] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, & Nixon K (2013). Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiology of Disease, 54, 239–251. 10.1016/j.nbd.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard ME, Barton EA, Robinson CR, Wooden JI, & Leasure JL (2018). Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder. Brain Structure & Function, 223(1), 195–210. 10.1007/s00429-017-1482-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath EL, Gao J, Kuo Y-F, Dunn TJ, Ray MJ, Dineley KT, Cunningham KA, Kaphalia BS, & Wu P (2017). Spatial and Sex-Dependent Responses of Adult Endogenous Neural Stem Cells to Alcohol Consumption. Stem Cell Reports, 9(6), 1916–1930. 10.1016/j.stemcr.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, & Marksteiner J (2007). A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. Journal of Neurology, Neurosurgery, and Psychiatry, 78(6), 610–614. 10.1136/jnnp.2006.095869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2006). Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol and Alcoholism, 41(4), 379–385. 10.1093/alcalc/agl006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2021). Astroglia in the vulnerability and maintenance of alcohol use disorders. Advances in Neurobiology, 26, 255–279. 10.1007/978-3-030-77375-5_11 [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, & Rajkowska G (2000). Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biological Psychiatry, 48(8), 861–873. 10.1016/S0006-3223(00)00999-9 [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, & Stockmeier CA (2010). Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. Journal of Affective Disorders, 127(1), 230–240. 10.1016/j.jad.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momenan R, Steckler LE, Saad ZS, van Rafelghem S, Kerich MJ, & Hommer DW (2012). Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Research: Neuroimaging, 204(2), 101–111. 10.1016/j.pscychresns.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, & Nixon K (2010). Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus, 20(5), 596–607. 10.1002/hipo.20665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, & Nixon K (2010). Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol (Fayetteville, N.Y.), 44(1), 89. 10.1016/j.alcohol.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Woodward JJ, & Chandler LJ (2009). Ethanol disrupts NMDA receptor and astroglial EAAT2 modulation of Kv2.1 potassium channels in hippocampus. Alcohol, 43(1), 45–50. 10.1016/j.alcohol.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, & Yesavage JA (1999). Gender differences in moderate drinking effects. Alcohol Research & Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism, 23(1), 55–64. [PMC free article] [PubMed] [Google Scholar]
- Nawarawong NN, Thompson KR, Guerin SP, Anasooya Shaji C, Peng H, & Nixon K (2021). Reactive, Adult Neurogenesis From Increased Neural Progenitor Cell Proliferation Following Alcohol Dependence in Female Rats. Frontiers in Neuroscience, 15, 689601. 10.3389/fnins.2021.689601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, & Crews FT (2004). Temporally Specific Burst in Cell Proliferation Increases Hippocampal Neurogenesis in Protracted Abstinence from Alcohol. Journal of Neuroscience, 24(43), 9714–9722. 10.1523/JNEUROSCI.3063-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, & Crews FT (2008). Distinct cell proliferation events during abstinence after alcohol dependence: Microglia proliferation precedes neurogenesis. Neurobiology of Disease, 31(2), 218–229. 10.1016/j.nbd.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, & Fornal CA (2011). The appropriateness of unbiased optical fractionators to assess cell proliferation in the adult hippocampus. Frontiers in Neuroscience, 5, 140. 10.3389/fnins.2011.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwachukwu KN, Evans WA, Sides TR, Trevisani CP, Davis A, & Marshall SA (2021). Chemogenetic manipulation of astrocytic signaling in the basolateral amygdala reduces binge-like alcohol consumption in male mice. Journal of Neuroscience Research, 99(8), 1957–1972. 10.1002/jnr.24841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwachukwu KN, King DM, Healey KL, Swartzwelder HS, & Marshall SA (2022). Sex-specific effects of adolescent intermittent ethanol exposure-induced dysregulation of hippocampal glial cells in adulthood. Alcohol (Fayetteville, N.Y.), 100, 31–39. 10.1016/j.alcohol.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, & Crews FT (2002). Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism, Clinical and Experimental Research, 26(4), 547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, & Crews FT (2002). Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, Biochemistry, and Behavior, 72(3), 521–532. 10.1016/s0091-3057(02)00715-3 [DOI] [PubMed] [Google Scholar]
- Pawlak J, Karolczak M, Krust A, Chambon P, & Beyer C (2005). Estrogen receptor-alpha is associated with the plasma membrane of astrocytes and coupled to the MAP/Src-kinase pathway. Glia, 50(3), 270–275. 10.1002/glia.20162 [DOI] [PubMed] [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallén A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, & Frisén J (1999). Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. The Journal of Cell Biology, 145(3), 503–514. 10.1083/jcb.145.3.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, & Nilsson M (2005). Astrocyte activation and reactive gliosis. Glia, 50(4), 427–434. 10.1002/glia.20207 [DOI] [PubMed] [Google Scholar]
- Peng H, Geil Nickell CR, Chen KY, McClain JA, & Nixon K (2017). Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol, 62, 29–40. 10.1016/j.alcohol.2017.02.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, & Nixon K (2020). Microglia Phenotype is Not as Simple as M1- or M2-like after Alcohol Dependence in Adolescent Rats. Alcoholism: Clinical and Experimental Research, n/a(n/a). 10.1111/acer.14504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Morgello S, Felix JC, & Lesser ML (1990). The two patterns of reactive astrocytosis in postischemic rat brain. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 10(6), 850–859. 10.1038/jcbfm.1990.141 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, & Sullivan EV (1992). Brain Gray and White Matter Volume Loss Accelerates with Aging in Chronic Alcoholics: A Quantitative MRI Study. Alcohol: Clinical and Experimental Research, 16(6), 1078–1089. 10.1111/j.1530-0277.1992.tb00702.x [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, & Sullivan E (2001). Sex differences in the effects of alcohol on brain structure. The American Journal of Psychiatry, 158(2), 188–197. 10.1176/appi.ajp.158.2.188 [DOI] [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, & Yeager RD (1989). Telescoping of alcoholism in women alcoholics. The International Journal of the Addictions, 24(1), 19–28. 10.3109/10826088909047272 [DOI] [PubMed] [Google Scholar]
- Poirier JL, Capek R, & De Koninck Y (2000). Differential progression of Dark Neuron and Fluoro-Jade labelling in the rat hippocampus following pilocarpine-induced status epilepticus. Neuroscience, 97(1), 59–68. 10.1016/s0306-4522(00)00026-9 [DOI] [PubMed] [Google Scholar]
- Potokar M, Morita M, Wiche G, & Jorgačevski J (2020). The Diversity of Intermediate Filaments in Astrocytes. Cells, 9(7). 10.3390/cells9071604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potokar M, Stenovec M, Gabrijel M, Li L, Kreft M, Grilc S, Pekny M, & Zorec R (2010). Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes. Glia, 58(10), 1208–1219. 10.1002/glia.21000 [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong J-S, & Crews FT (2008). Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of Neuroinflammation, 5(1), 10. 10.1186/1742-2094-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PSS, Bell RL, Engleman EA, & Sari Y (2015). Targeting glutamate uptake to treat alcohol use disorders. Frontiers in Neuroscience, 9. 10.3389/fnins.2015.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Tello M, Vallés S, Montoliu C, Renau-Piqueras J, & Guerri C (1995). Developmental pattern of GFAP and vimentin gene expression in rat brain and in radial glial cultures. Glia, 15(2), 157–166. 10.1002/glia.440150208 [DOI] [PubMed] [Google Scholar]
- Sarkisyan D, Bazov I, Watanabe H, Kononenko O, Syvänen A-C, Schumann G, Yakovleva T, & Bakalkin G (2017). Damaged reward areas in human alcoholics: Neuronal proportion decline and astrocyte activation. Acta Neuropathologica, 133(3), 485–487. 10.1007/s00401-017-1675-0 [DOI] [PubMed] [Google Scholar]
- Sawyer KS, Oscar-Berman M, Barthelemy OJ, Papadimitriou GM, Harris GJ, & Makris N (2017). Gender dimorphism of brain reward system volumes in alcoholism. Psychiatry Research. Neuroimaging, 263, 15–25. 10.1016/j.pscychresns.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer KS, Oscar-Berman M, Mosher Ruiz S, Gálvez DA, Makris N, Harris GJ, & Valera EM (2016). Associations Between Cerebellar Subregional Morphometry and Alcoholism History in Men and Women. Alcoholism, Clinical and Experimental Research, 40(6), 1262–1272. 10.1111/acer.13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, & Cardona A (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Szymas J, & Hossmann K-A (1990). Immunohistochemical study of glial reaction and serum-protein extravasation in relation to neuronal damage in rat hippocampus after ischemia. Neuroscience, 38(2), 527–540. 10.1016/0306-4522(90)90048-9 [DOI] [PubMed] [Google Scholar]
- Schmued LC, & Hopkins KJ (2000). Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Research, 874(2), 123–130. 10.1016/S0006-8993(00)02513-0 [DOI] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, & Xu L (2005). Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Research, 1035(1), 24–31. 10.1016/j.brainres.2004.11.054 [DOI] [PubMed] [Google Scholar]
- Schnitzer J, Franke W, & Schachner M (1981). Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. The Journal of Cell Biology, 90(2), 435–447. 10.1083/jcb.90.2.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade T, Chapman C, Swift W, Keyes K, Tonks Z, & Teesson M (2016). Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: Systematic review and metaregression. BMJ Open, 6(10), e011827. 10.1136/bmjopen-2016-011827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TL (1997). Regulation of glutamate uptake in astrocytes continuously exposed to ethanol. Life Sciences, 61(25), 2499–2505. 10.1016/S0024-3205(97)00985-5 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, & Vinters HV (2010). Astrocytes: Biology and pathology. Acta Neuropathologica, 119(1), 7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, & Potvin S (2013). Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addiction Biology, 18(2), 203–213. 10.1111/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, & Pfefferbaum A (2002). A profile of neuropsychological deficits in alcoholic women. Neuropsychology, 16(1), 74–83. 10.1037//0894-4105.16.1.74 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2005). Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology, 180(4), 583–594. 10.1007/s00213-005-2267-6 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, & Pfefferbaum A (2010). Pontocerebellar volume deficits and ataxia in alcoholic men and women: No evidence for “telescoping.” Psychopharmacology, 208(2), 279–290. 10.1007/s00213-009-1729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, & Pfefferbaum A (2000). Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism, Clinical and Experimental Research, 24(5), 611–621. [PubMed] [Google Scholar]
- Thayer RE, Hagerty SL, Sabbineni A, Claus ED, Hutchison KE, & Weiland BJ (2016). Negative and interactive effects of sex, aging, and alcohol abuse on gray matter morphometry. Human Brain Mapping, 37(6), 2276–2292. 10.1002/hbm.23172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, Gabrijel M, Potokar M, Svajger U, Kreft M, Jeras M, de Pablo Y, Faiz M, Pekny M, & Zorec R (2012). IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. Journal of Neuroinflammation, 9, 144. 10.1186/1742-2094-9-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, & Nedergaard M (2018). Physiology of Astroglia. Physiological Reviews, 98(1), 239–389. 10.1152/physrev.00042.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Cosgrove KP, Tanabe J, & McKee SA (2021). Sex/gender differences in brain function and structure in alcohol use: A narrative review of neuroimaging findings over the last 10 years. Journal of Neuroscience Research, 99(1), 309–323. 10.1002/jnr.24625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, & Kubanis P (1980). Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science (New York, N.Y.), 209(4457), 711–713. 10.1126/science.7394532 [DOI] [PubMed] [Google Scholar]
- West RK, Rodgers SP, & Leasure JL (2021). Neural Perturbations Associated With Recurrent Binge Alcohol in Male and Female Rats. Alcohol: Clinical and Experimental Research, 45(2), 365–374. 10.1111/acer.14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM (2020). Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Research : Current Reviews, 40(2), 01. 10.35946/arcr.v40.2.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR, & Wiren KM (2016). Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathology, 26(4), 433–451. 10.1111/bpa.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Beard DK, & Wiren KM (2015). Females uniquely vulnerable to alcohol-induced neurotoxicity show altered glucocorticoid signaling. Brain Research, 1601, 102–116. 10.1016/j.brainres.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Kawamata T, Walker DG, & McGeer PL (1992). Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathologica, 84(2), 157–162. 10.1007/BF00311389 [DOI] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV, & Pfefferbaum A (2010). Measurement of Serum, Liver, and Brain Cytokine Induction, Thiamine Levels, and Hepatopathology in Rats Exposed to a 4-Day Alcohol Binge Protocol. Alcohol: Clinical and Experimental Research, 34(11), 1858–1870. 10.1111/j.1530-0277.2010.01274.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, & Constable RT (2017). Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. NeuroImage. Clinical, 13, 181–187. 10.1016/j.nicl.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Pohl KM, Sullivan EV, Pfefferbaum A, & Zahr NM (2021). Jacobian Mapping Reveals Converging Brain Substrates of Disruption and Repair in Response to Ethanol Exposure and Abstinence in 2 Strains of Rats. Alcohol: Clinical and Experimental Research, 45(1), 92–104. 10.1111/acer.14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwain IH, & Yen SS (1999). Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology, 140(8), 3843–3852. 10.1210/endo.140.8.6907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Additional images of FJB stained tissue not shown in Figure 2. Controls for each time point and an additional region, the posterolateral cortical amygdaloid nucleus (PLCo) are shown. Representative images are taken at the T0, T2, T7 and T14 time points. Scale bar = 50μm.
Supplementary Figure 2: Additional images of vimentin immunoreactivity in cortical regions not shown in Figure 5. Controls for each time point and an additional region, the auditory cortex, are shown. Representative images are taken at the T2, T7 and T14 time points. Scale bar = 250 μm.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


