Abstract
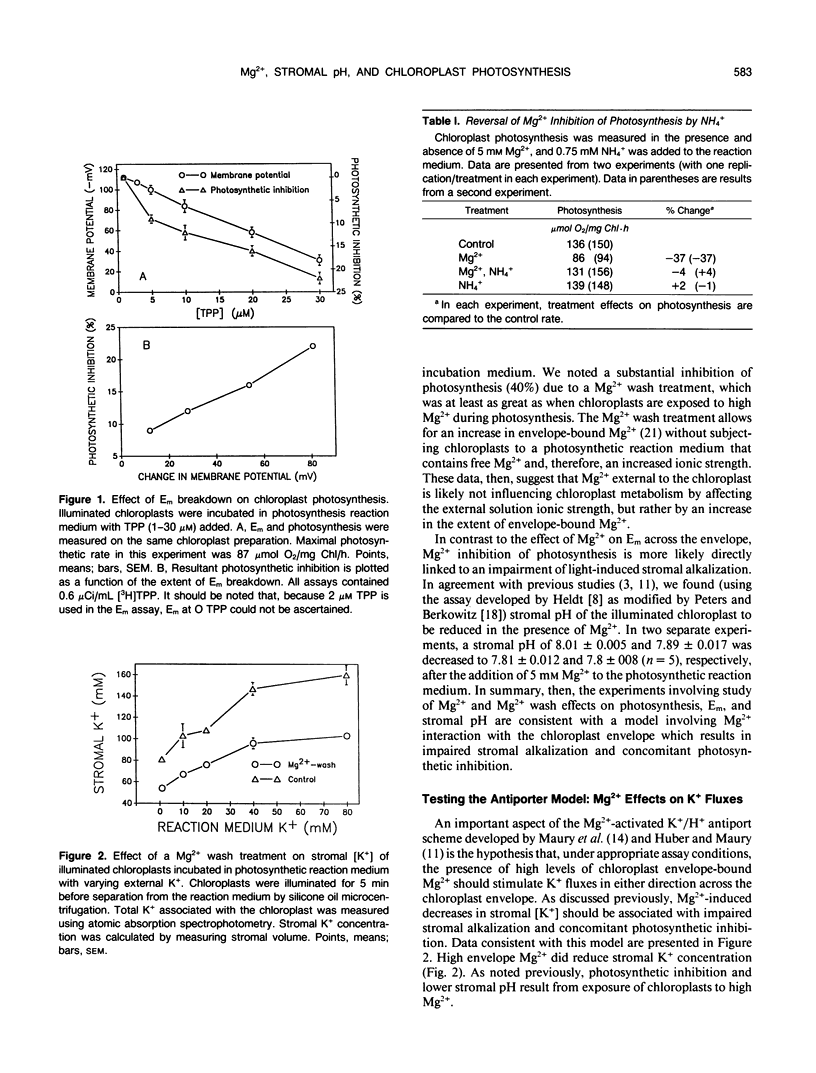
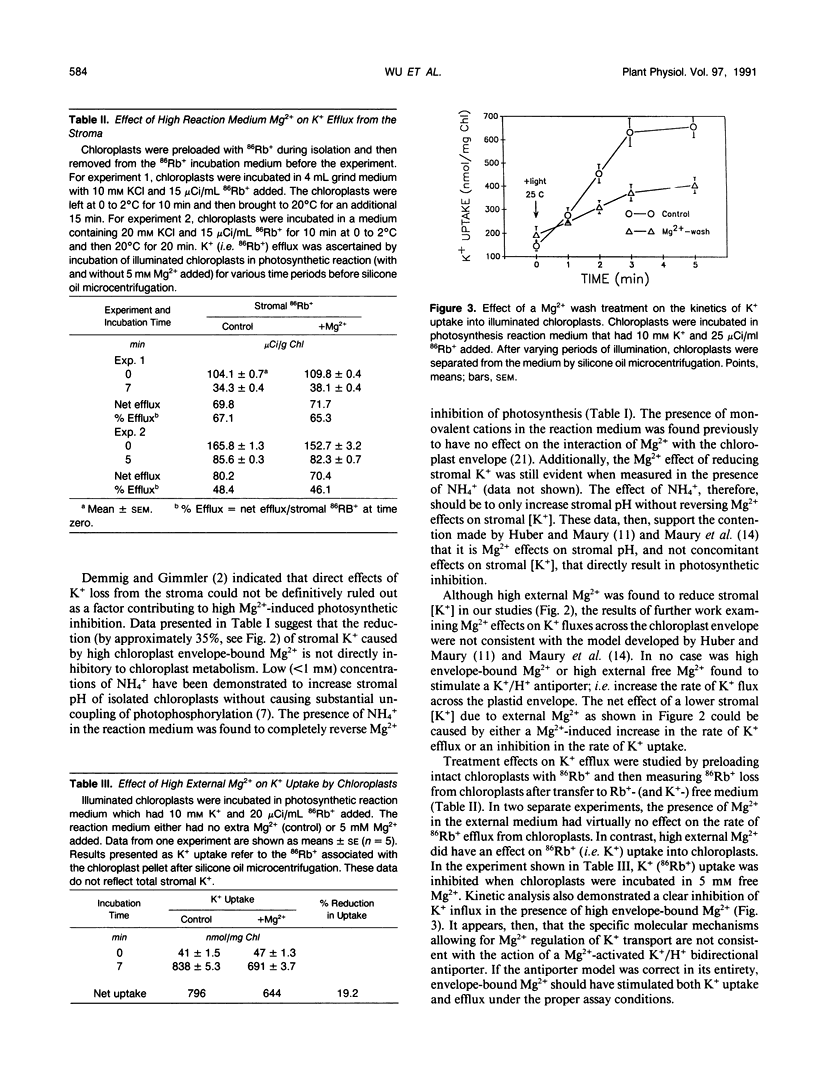
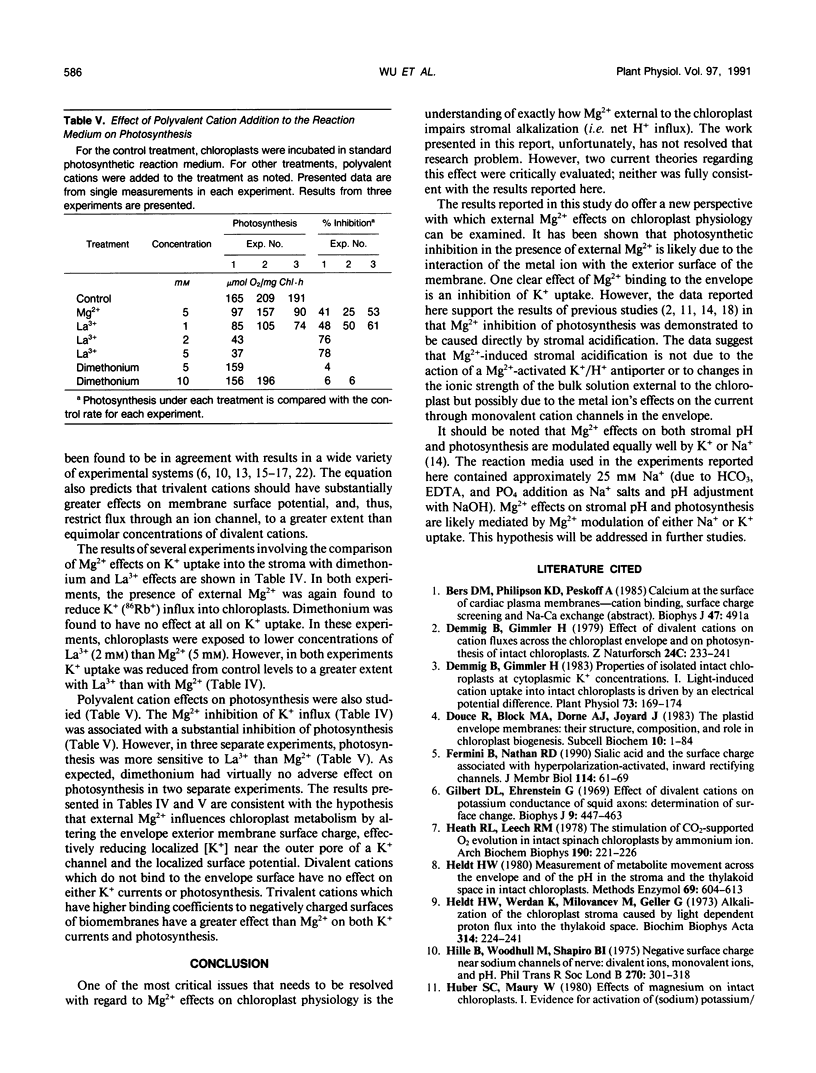
Studies of Spinacia oleracea L. were undertaken to characterize further how Mg2+ external to the isolated intact chloroplast interacts with stromal K+, pH, and photosynthetic capacity. Data presented in this report were consistent with the previously developed hypothesis that millimolar levels of external, unchelated Mg2+ result in lower stromal K+, which somehow is linked to stromal acidification. Stromal acidification directly results in photosynthetic inhibition. These effects were attributed to Mg2+ interaction (binding) to negative surface charges on the chloroplast envelope. Chloroplast envelope-bound Mg2+ was found to decrease the envelope membrane potential (inside negative) of the illuminated chloroplast by 10 millivolts. It was concluded that Mg2+ effects on photosynthesis were likely not mediated by this effect on membrane potential. Further experiments indicated that envelope-bound Mg2+ caused lower stromal K+ by restricting the rate of K+ influx; Mg2+ did not affect K+ efflux from the stroma. Mg2+ restriction of K+ influx appeared consistent with the typical effects imposed on monovalent cation channels by polyvalent cations that bind to negatively charged sites on a membrane surface near the outer pore of the channel. It was hypothesized that this interaction of Mg2+ with the chloroplast envelope likely mediated external Mg2+ effects on chloroplast metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demmig B., Gimmler H. Properties of the Isolated Intact Chloroplast at Cytoplasmic K Concentrations : I. Light-Induced Cation Uptake into Intact Chloroplasts is Driven by an Electrical Potential Difference. Plant Physiol. 1983 Sep;73(1):169–174. doi: 10.1104/pp.73.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Block M. A., Dorne A. J., Joyard J. The plastid envelope membranes: their structure, composition, and role in chloroplast biogenesis. Subcell Biochem. 1984;10:1–84. doi: 10.1007/978-1-4613-2709-7_1. [DOI] [PubMed] [Google Scholar]
- Fermini B., Nathan R. D. Sialic acid and the surface charge associated with hyperpolarization-activated, inward rectifying channels. J Membr Biol. 1990 Mar;114(1):61–69. doi: 10.1007/BF01869385. [DOI] [PubMed] [Google Scholar]
- Gilbert D. L., Ehrenstein G. Effect of divalent cations on potassium conductance of squid axons: determination of surface charge. Biophys J. 1969 Mar;9(3):447–463. doi: 10.1016/S0006-3495(69)86396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. S., Berkowitz G. A. Development and use of chlorotetracycline fluorescence as a measurement assay of chloroplast envelope-bound mg. Plant Physiol. 1989 Mar;89(3):753–761. doi: 10.1104/pp.89.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R. L., Leech R. M. The stimulation of CO2-supported O2 evolution in intact spinach chloroplasts by ammonium ion. Arch Biochem Biophys. 1978 Sep;190(1):221–226. doi: 10.1016/0003-9861(78)90271-0. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Maury W. Effects of Magnesium on Intact Chloroplasts: I. EVIDENCE FOR ACTIVATION OF (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE CHLOROPLAST ENVELOPE. Plant Physiol. 1980 Feb;65(2):350–354. doi: 10.1104/pp.65.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury W. J., Huber S. C., Moreland D. E. Effects of Magnesium on Intact Chloroplasts : II. CATION SPECIFICITY AND INVOLVEMENT OF THE ENVELOPE ATPase IN (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE ENVELOPE. Plant Physiol. 1981 Dec;68(6):1257–1263. doi: 10.1104/pp.68.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin A., Eng W. K., Vaio G., Wilson T., McLaughlin S. Dimethonium, a divalent cation that exerts only a screening effect on the electrostatic potential adjacent to negatively charged phospholipid bilayer membranes. J Membr Biol. 1983;76(2):183–193. doi: 10.1007/BF02000618. [DOI] [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P. Effect of surface charge on the steady-state potassium conductance of nodal membrane. Nature. 1970 Oct 10;228(5267):164–165. doi: 10.1038/228164a0. [DOI] [PubMed] [Google Scholar]
- Peters J. S., Berkowitz G. A. Studies on the System Regulating Proton Movement across the Chloroplast Envelope : Effects of ATPase Inhibitors, Mg, and an Amine Anesthetic on Stromal pH and Photosynthesis. Plant Physiol. 1991 Apr;95(4):1229–1236. doi: 10.1104/pp.95.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Prasad R., Höfer M. Tetraphenylphosphonium is an indicator of negative membrane potential in Candida albicans. Biochim Biophys Acta. 1986 Oct 9;861(2):377–380. doi: 10.1016/0005-2736(86)90442-6. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. Intracellular Mg2+ may act as a co-factor in ion channel function. Trends Neurosci. 1988 Nov;11(11):475–477. doi: 10.1016/0166-2236(88)90003-3. [DOI] [PubMed] [Google Scholar]


