Abstract
Acetohydroxyacid synthase (AHAS, EC 4.1.3.18) is the first enzyme unique to the biosynthesis of valine, leucine, and isoleucine. This enzyme is the target site of several classes of structurally unrelated herbicides. The conventional method of antibody production using purified protein has not been successful with this enzyme. Two separate fragments of a gene encoding a portion of the mature region of AHAS from Arabidopsis were fused with the trpE gene from Escherichia coli using the pATH1 vector. E. coli cells transformed with each respective plasmid expressed a fusion protein at levels greater than 10% of the total cell protein. The fusion protein was purified and used to immunize rabbits. Antisera obtained from the immunized rabbits immunoprecipitated AHAS activity from Arabidopsis cell free extracts. The anti-AHAS antisera reacted with a 65 kilodalton protein band in electrophoretically resolved extracts of Arabidopsis. In cross-reactivity tests, this antibody was able to immunoprecipitate AHAS activity from various plant species. Furthermore, a protein band with a molecular mass of 65 kilodaltons was detected in the crude extracts of all plant species tested on a Western blot. These results indicate that the 65 kilodalton protein represents AHAS in various plant species. The wide spectrum of cross-reactivity for the antisera supports the view that the AHAS enzyme is highly conserved across all plant species.
Full text
PDF



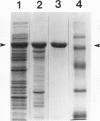
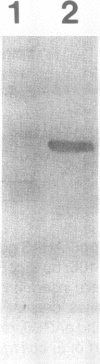
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaleff R. S., Mauvais C. J. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science. 1984 Jun 29;224(4656):1443–1445. doi: 10.1126/science.224.4656.1443. [DOI] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- LaRossa R. A., Schloss J. V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J Biol Chem. 1984 Jul 25;259(14):8753–8757. [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F., Smith J. K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987 Dec;85(4):1110–1117. doi: 10.1104/pp.85.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J. Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine, and valine. Arch Biochem Biophys. 1971 Oct;146(2):542–550. doi: 10.1016/0003-9861(71)90159-7. [DOI] [PubMed] [Google Scholar]
- Miflin B. J. The location of nitrite reductase and other enzymes related to amino Acid biosynthesis in the plastids of root and leaves. Plant Physiol. 1974 Oct;54(4):550–555. doi: 10.1104/pp.54.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhitch M. J., Shaner D. L., Stidham M. A. Imidazolinones and acetohydroxyacid synthase from higher plants: properties of the enzyme from maize suspension culture cells and evidence for the binding of imazapyr to acetohydroxyacid synthase in vivo. Plant Physiol. 1987 Feb;83(2):451–456. doi: 10.1104/pp.83.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ray T. B. Site of action of chlorsulfuron: inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984 Jul;75(3):827–831. doi: 10.1104/pp.75.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner D. L., Anderson P. C., Stidham M. A. Imidazolinones: potent inhibitors of acetohydroxyacid synthase. Plant Physiol. 1984 Oct;76(2):545–546. doi: 10.1104/pp.76.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. K., Stidham M. A., Shaner D. L. Assay of acetohydroxyacid synthase. Anal Biochem. 1988 May 15;171(1):173–179. doi: 10.1016/0003-2697(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- Wiersma P. A., Schmiemann M. G., Condie J. A., Crosby W. L., Moloney M. M. Isolation, expression and phylogenetic inheritance of an acetolactate synthase gene from Brassica napus. Mol Gen Genet. 1989 Nov;219(3):413–420. doi: 10.1007/BF00259614. [DOI] [PubMed] [Google Scholar]