Abstract
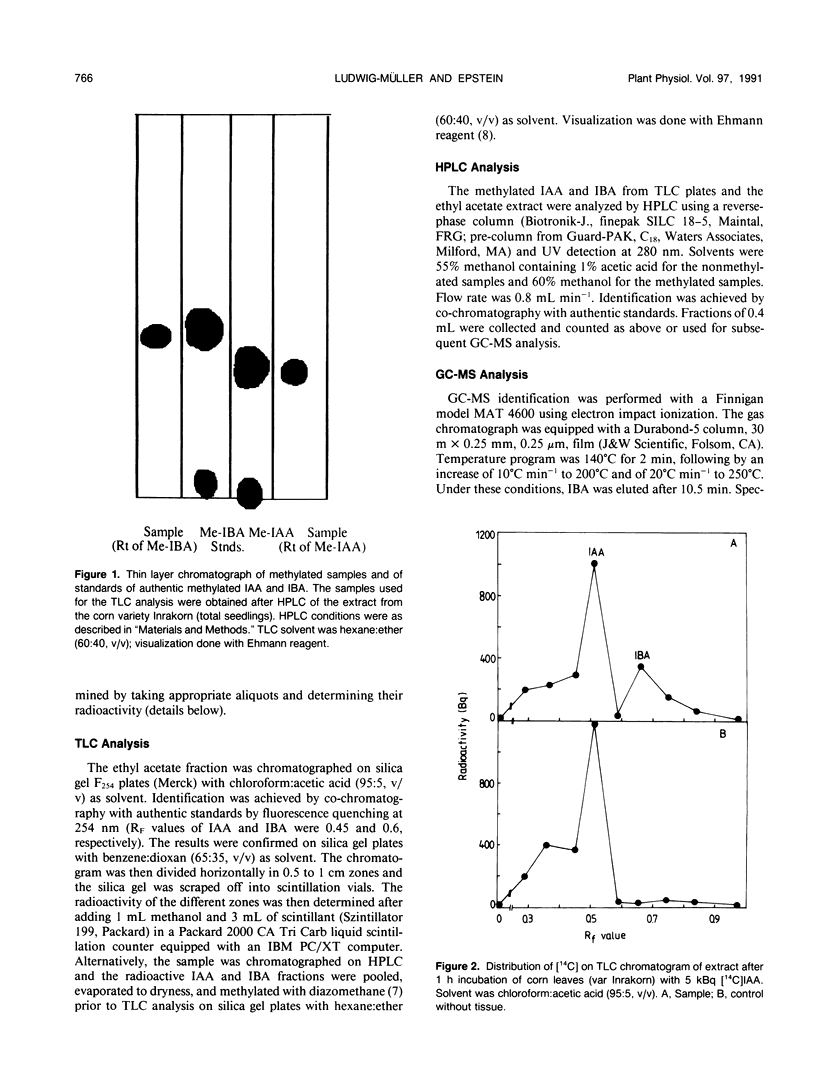
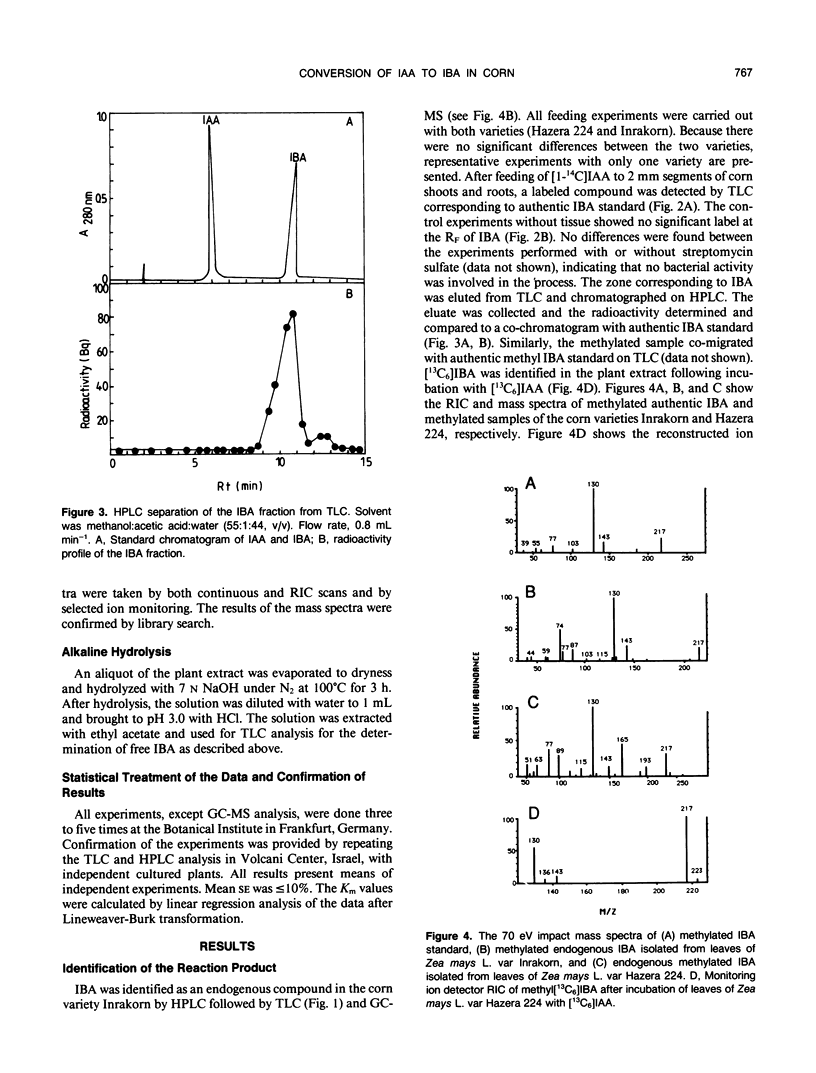
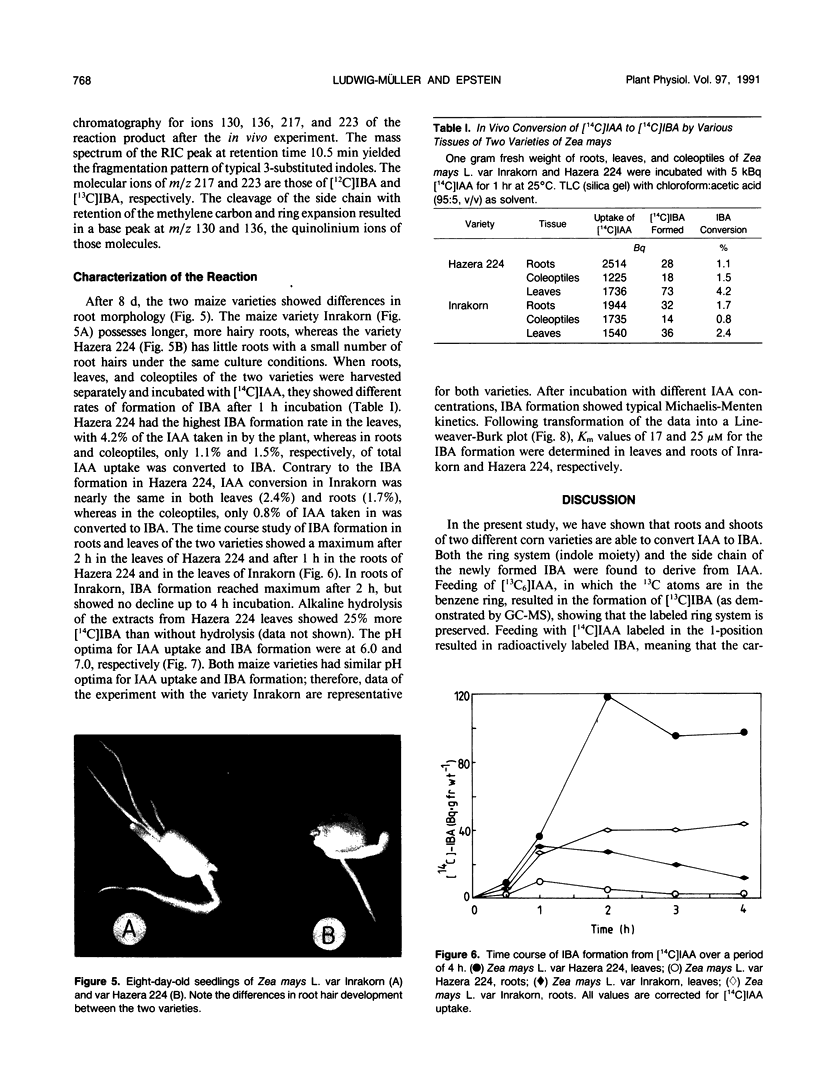
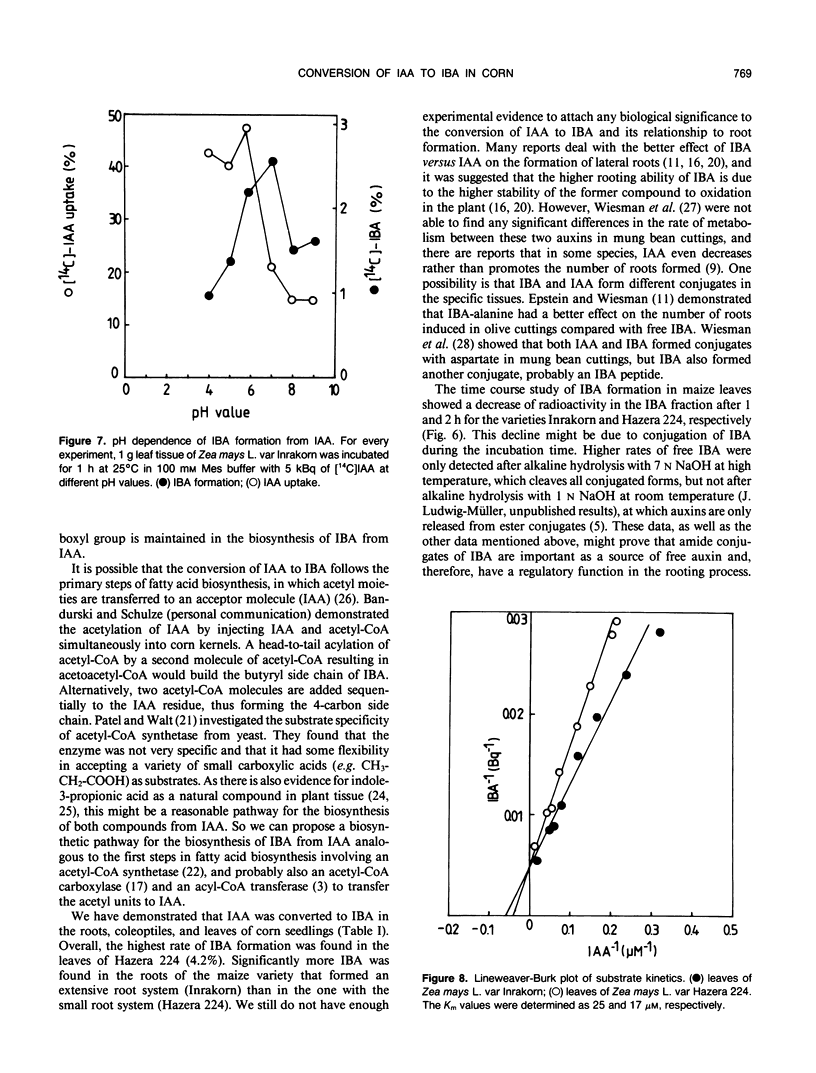
Indole-3-butyric acid (IBA) was identified as an endogenous compound in leaves and roots of maize (Zea mays L.) var Inrakorn by thin layer chromatography, high-performance liquid chromatography, and gas chromatography-mass spectrometry. Its presence was also confirmed in the variety Hazera 224. Indole-3-acetic acid (IAA) was metabolized to IBA in vivo by seedlings of the two maize varieties. The reaction product was identified by thin layer chromatography, high performance liquid chromatography, and gas chromatography-mass spectrometry after incubating the corn seedlings with [14C]IAA and [13C6]IAA. The in vivo conversion of IAA to IBA and the characteristics of IBA formation in two different maize varieties of Zea mays L. (Hazera 224 and Inrakorn) were investigated. IBA-forming activity was examined in the roots, leaves, and coleoptiles of both maize varieties. Whereas in the variety Hazera 224, IBA was formed mostly in the leaves, in the variety Inrakorn, IBA synthesis was detected in the roots as well as in the leaves. A time course study of IBA formation showed that maximum activity was reached in Inrakorn after 1 hour and in Hazera after 2 hours. The pH optimum for the uptake of IAA was 6.0, and that for IBA formation was 7.0. The Km value for IBA formation was 17 micromolar for Inrakorn and 25 micromolar for Hazera 224. The results are discussed with respect to the possible functions of IBA in the plant.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae W. A., Good N. E. Studies on 3-Indoleacetic Acid Metabolism. IV. Conjugation with Aspartic Acid and Ammonia as Processes in the Metabolism of Carboxylic Acids. Plant Physiol. 1957 Nov;32(6):566–572. doi: 10.1104/pp.32.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Cohen J. D. Free and conjugated indole-3-acetic Acid in developing bean seeds. Plant Physiol. 1989 Oct;91(2):775–779. doi: 10.1104/pp.91.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Cohen J. D. Isolation and Partial Characterization of the Major Amide-Linked Conjugate of Indole-3-Acetic Acid from Phaseolus vulgaris L. Plant Physiol. 1986 Jan;80(1):99–104. doi: 10.1104/pp.80.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- FAWCETT C. H., WAIN R. L., WIGHTMAN F. The metabolism of 3-indolylalkanecarboxylic acids, and their amides, nitriles and methyl esters in plant tissues. Proc R Soc Lond B Biol Sci. 1960 May 17;152:231–254. doi: 10.1098/rspb.1960.0035. [DOI] [PubMed] [Google Scholar]
- NISHIDA S. Potent diphtheria toxin within the cells of C. diphtheriae. Nature. 1954 Nov 20;174(4438):970–970. doi: 10.1038/174970a0. [DOI] [PubMed] [Google Scholar]
- Patel S. S., Walt D. R. Substrate specificity of acetyl coenzyme A synthetase. J Biol Chem. 1987 May 25;262(15):7132–7134. [PubMed] [Google Scholar]
- Preston G. G., Wall J. D., Emerich D. W. Purification and properties of acetyl-CoA synthetase from Bradyrhizobium japonicum bacteroids. Biochem J. 1990 Apr 1;267(1):179–183. doi: 10.1042/bj2670179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil S. J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989 May 30;28(11):4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- Wiesman Z., Riov J., Epstein E. Characterization and Rooting Ability of Indole-3-Butyric Acid Conjugates Formed during Rooting of Mung Bean Cuttings. Plant Physiol. 1989 Nov;91(3):1080–1084. doi: 10.1104/pp.91.3.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]



