Abstract
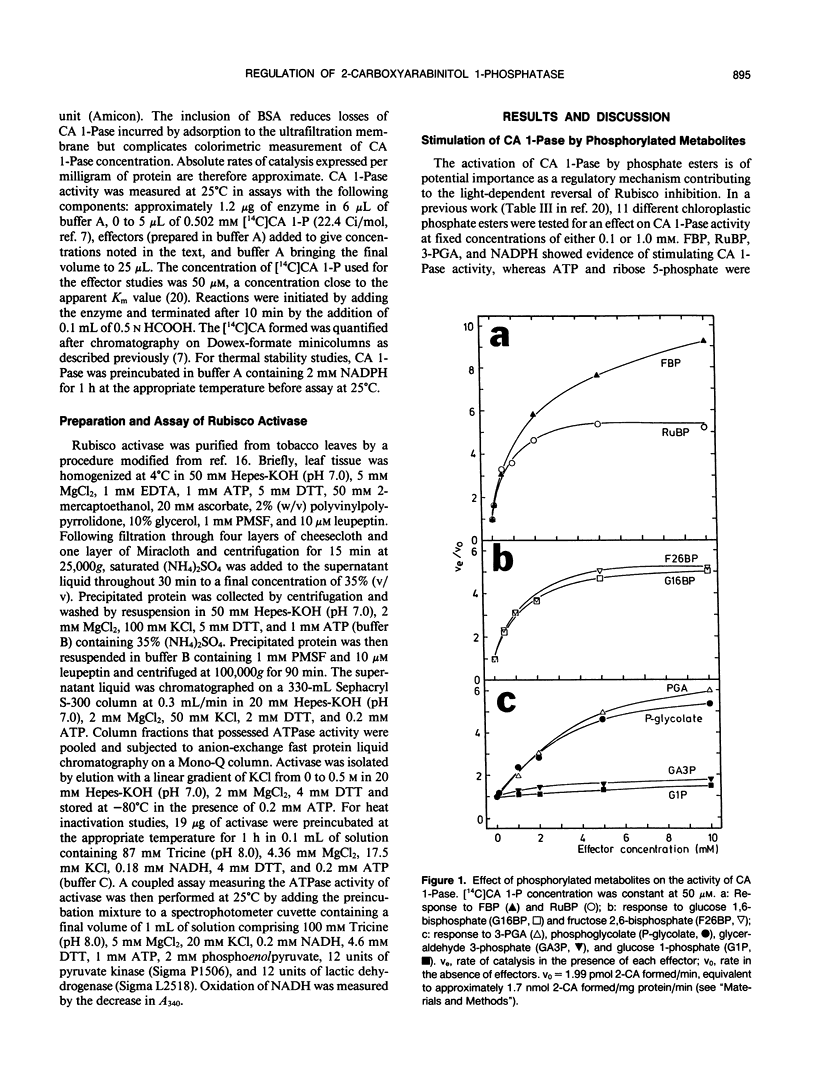
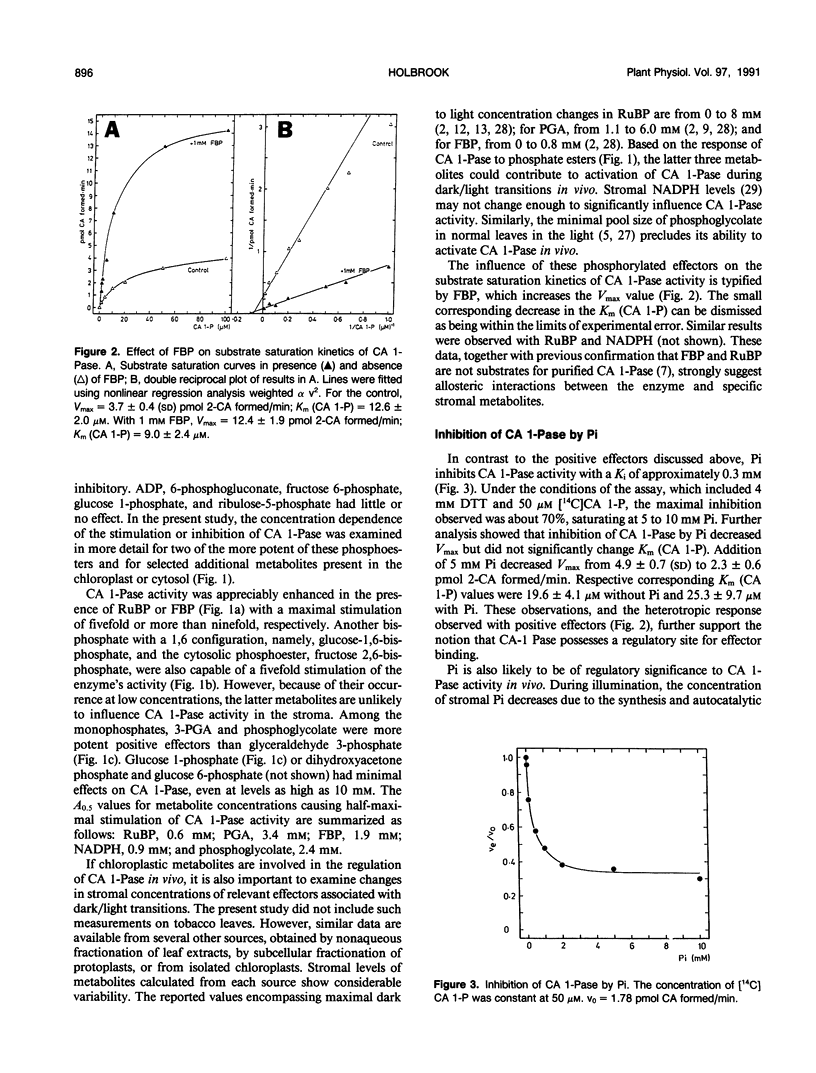
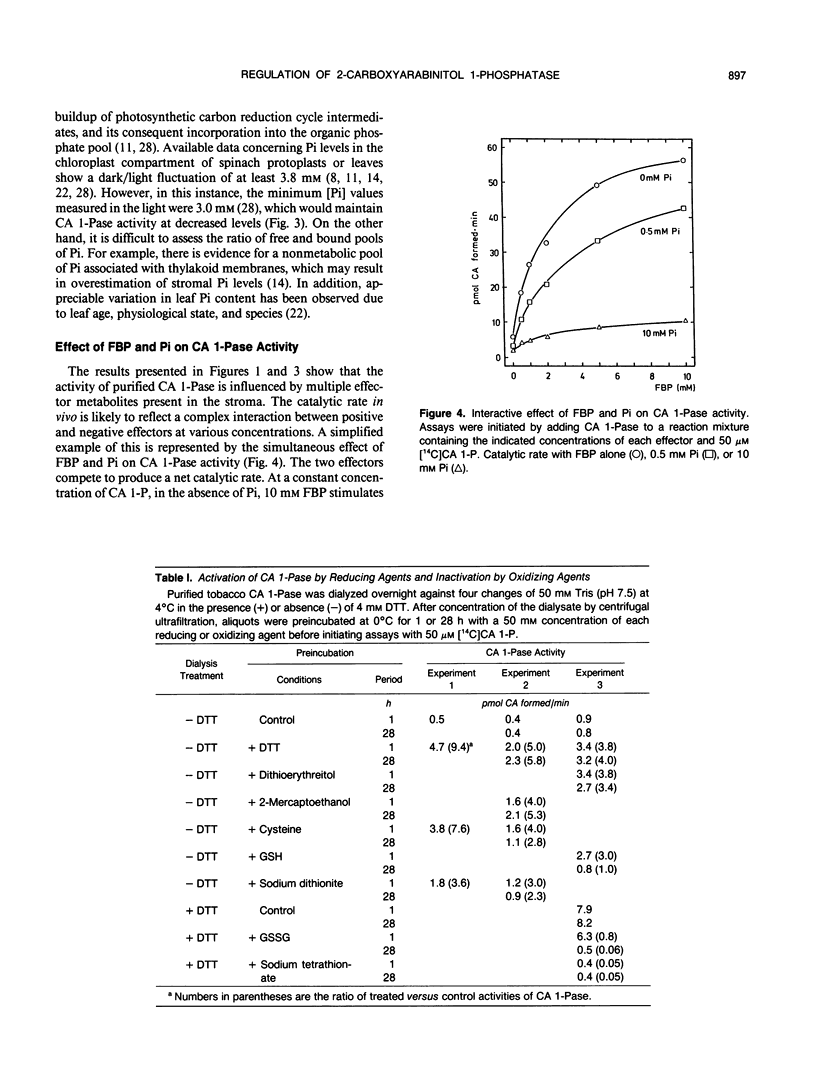
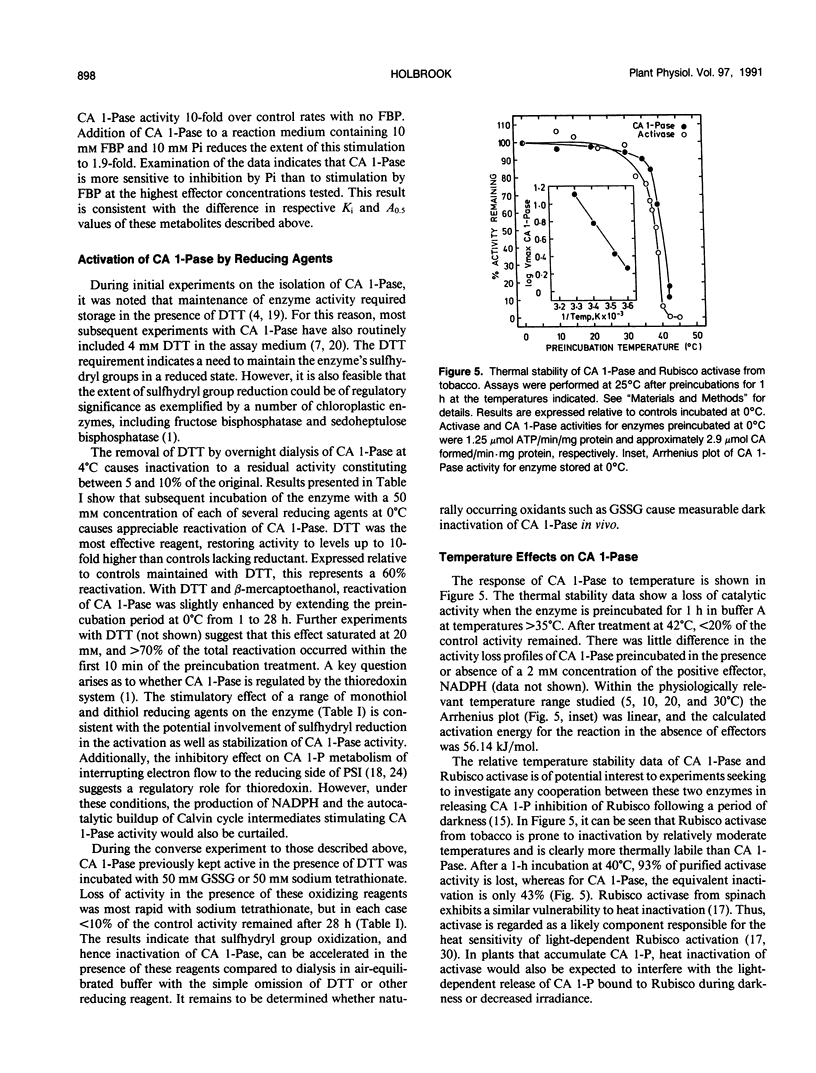
The regulation of 2-carboxyarabinitol 1-phosphatase (CA 1-Pase) by phosphorylated effectors was studied with enzyme purified from tobacco (Nicotiana tabacum) leaves. CA 1-Pase activity was most stimulated by fructose 1,6-bisphosphate, exhibiting an A0.5 value of 1.9 millimolar and a 10-fold enhancement of catalysis. With ribulose-1,5-bisphosphate, the A0.5 was 0.6 millimolar, and maximal stimulation of activity was 5.3-fold. Among the monophosphates, 3-phosphoglycerate and phosphoglycolate were more potent positive effectors than glyceraldehyde 3-phosphate, glucose 1-phosphate, glucose 6-phosphate, and dihydroxyacetone phosphate. Stimulation of CA 1-Pase by ribulose-1,5-bisphosphate and fructose 1,6-bisphosphate increased Vmax but did not appreciably alter Km (2-carboxyarabinitol 1-phosphate) values. Inorganic phosphate appeared to inhibit CA 1-Pase noncompetitively with respect to 2-carboxyarabinitol 1-phosphate, exhibiting a Ki of 0.3 millimolar. The results suggest that these positive and negative effectors bind to a regulatory site on CA 1-Pase and may have a physiologial role in the light regulation of this enzyme. Related experiments with CA 1-Pase inactivated by dialysis in the absence of dithiothreitol show that partial reactivation can be achieved in the presence of a range of reducing reagents, including dithiothreitol, cysteine, and reduced glutathione. This could imply an ancillary involvement of sulfhydryl reduction during light activation of CA 1-Pase in vivo. The enzyme was thermally stable up to 35°C, in contrast to ribulose-1,5-bisphosphate carboxylase/oxygenase activase which lost activity above 30°C. The activation energy for CA 1-Pase was calculated to be 56.14 kilojoules per mole.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Holbrook G. P., Bowes G., Salvucci M. E. Degradation of 2-carboxyarabinitol 1-phosphate by a specific chloroplast phosphatase. Plant Physiol. 1989 Jun;90(2):673–678. doi: 10.1104/pp.90.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Bassham J. A. Light-Dark Regulation of Starch Metabolism in Chloroplasts: I. Levels of Metabolites in Chloroplasts and Medium during Light-Dark Transition. Plant Physiol. 1979 Jan;63(1):105–108. doi: 10.1104/pp.63.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W., Urbach W. The effect of dihydroxyacetone phosphate and 3-phosphoglycerate on O2 evolution and on the levels of ATP, ADP and Pi in isolated intact chloroplasts. Biochim Biophys Acta. 1977 Mar 11;459(3):337–346. doi: 10.1016/0005-2728(77)90035-4. [DOI] [PubMed] [Google Scholar]
- Kobza J., Seemann J. R. Light-dependent kinetics of 2-carboxyarabinitol 1-phosphate metabolism and ribulose-1,5-bisphosphate carboxylase activity in vivo. Plant Physiol. 1989 Jan;89(1):174–179. doi: 10.1104/pp.89.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Chon C. J., Mosbach A., Heldt H. W. The distribution of metabolites between spinach chloroplasts and medium during photosynthesis in vitro. Biochim Biophys Acta. 1977 May 11;460(2):259–272. doi: 10.1016/0005-2728(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Marques I. A., Ford D. M., Muschinek G., Anderson L. E. Photosynthetic carbon metabolism in isolated pea chloroplasts: metabolite levels and enzyme activities. Arch Biochem Biophys. 1987 Feb 1;252(2):458–466. doi: 10.1016/0003-9861(87)90052-x. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R., Jr Adenosine triphosphate hydrolysis by purified rubisco activase. Arch Biochem Biophys. 1989 Jan;268(1):93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Streusand V. J., Chatfield J. M., Portis A. R. Purification and assay of rubisco activase from leaves. Plant Physiol. 1988 Dec;88(4):1008–1014. doi: 10.1104/pp.88.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Anderson J. C. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987 Sep;85(1):66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Holbrook G. P. Purification and Properties of 2-Carboxy-d-Arabinitol 1-Phosphatase. Plant Physiol. 1989 Jun;90(2):679–685. doi: 10.1104/pp.90.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Seemann J. R., Berry J. A., Freas S. M., Krump M. A. Regulation of ribulose bisphosphate carboxylase activity in vivo by a light-modulated inhibitor of catalysis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8024–8028. doi: 10.1073/pnas.82.23.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Inhibition of ribulose 1,5-bisphosphate carboxylase/oxygenase by 2-carboxyarabinitol-1-phosphate. Plant Physiol. 1990 Apr;92(4):867–870. doi: 10.1104/pp.92.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Parry M. A., Gutteridge S., Keys A. J. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986 Dec;82(4):1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta. 1980 Nov 5;593(1):85–102. doi: 10.1016/0005-2728(80)90010-9. [DOI] [PubMed] [Google Scholar]


