Abstract
INTRODUCTION:
We used sex and apolipoprotein E ε4 (APOE-ε4) carrier status as predictors of pathologic burden in early-onset Alzheimer’s disease (EOAD).
METHODS:
We included baseline data from 77 cognitively normal (CN), 230 EOAD and 70 EO non-Alzheimer’s disease (EOnonAD) participants from the Longitudinal Early-Onset Alzheimer’s Disease Study (LEADS). We stratified each diagnostic group by males and females, then further subdivided each sex by APOE-ε4 carrier status and compared imaging biomarkers in each stratification. Voxel-wise multiple linear regressions yielded statistical brain maps of gray matter density, amyloid and tau PET burden.
RESULTS:
EOAD females had greater amyloid and tau PET burden than males. EOAD female APOE-ε4 non-carriers had greater amyloid PET burden and greater gray matter atrophy than female ε4 carriers. EOnonAD female ε4 non-carriers also had greater gray matter atrophy than female ε4 carriers.
DISCUSSION:
The effects of sex and APOE-ε4 must be considered when studying these populations.
Keywords: Sex differences, APOE-ε4, Early-onset Alzheimer’s disease, Early-onset non-Alzheimer’s disease, genetics, neuroimaging, MRI, amyloid PET, tau PET, imaging biomarkers
1. BACKGROUND
Sporadic Alzheimer’s disease (AD) is a heterogeneous, multifactorial disorder that presents with a high degree of clinicopathological variability across the AD spectrum. While the morphological hallmarks of extracellular amyloid-β and intraneuronal tau aggregates forming neurofibrillary tangles can be observed across different variants, disease onset, trajectory, and clinical phenotype are highly variable1.
The early-onset AD (EOAD; onset <65 years) variant is an atypical, insidious, and aggressive form of AD with an accelerated trajectory of cognitive decline and greater pathology burden compared to late-onset AD (LOAD)2–5. EOAD manifests with more extensive neurodegeneration and tau burden in regions classically correlated with AD. Neuroimaging measures reveal greater global atrophy on magnetic-resonance imaging (MRI), extensive hypometabolism in the bilateral parietal, temporal, and frontal lobes, as well as insular and cingulate cortices on fluorodeoxyglucose-positron emission tomography (FDG-PET) compared to cognitively normal (CN) controls, with a stronger effect size in EOAD vs. LOAD6.
A multitude of factors, including biological sex and certain genes, modulate lifetime AD risk and disease phenotype. When considering sex differences, longitudinal studies examining sex in LOAD and EOAD suggest that female participants are subject to faster disease progression, greater pathology burden and seem to be more susceptible to clinical manifestations of AD than male participants7–9. Female EOAD participants presented with greater global amyloid-β burden and greater tau burden in the frontal, inferior parietal, and temporal lobes, along with more pronounced hippocampal atrophy10.
While most patients with a clinical diagnosis of AD are positive on amyloid-β scans, 15–35% of clinically diagnosed AD patients are found to be amyloid-β negative11–13. These individuals exhibit evidence of neurodegeneration, but do not meet the biomarker threshold for brain amyloidosis14–16. As a result, they are classified as non-AD or early-onset non-AD (EOnonAD)11–13. EOnonAD dementia is caused by multiple etiologies, presenting as heterogenous clinical trajectories and outcomes11. Chételat et al.11, found EOnonAD populations to be a mix of limbic-predominant pathologies that largely affect the medial temporal lobe, which makes the identification of succinct patterns of atrophy and tau PET deposition difficult5.
Among those diagnosed with AD, the prevalence of apolipoprotein E ε4 (APOE- ε4) is upwards of 40%, compared to between 13–23% in non-AD individuals worldwide17. Compared to other isoforms, the APOE-ε4 variant is associated with greater amyloid-β deposition in the brain18,19. Tau-mediated neurodegeneration may also be markedly exacerbated by APOE-ε4 leading to increased tau deposition20,21. APOE-ε4 is associated with earlier symptom onset in LOAD, and possibly a later symptom onset in EOAD, though it appears the ε4 allele has maximum impact between the onset ages of 60–70 years4,22,23. Further, Polsinelli et al.4, found female EOAD APOE-ε4 non-carriers had significantly younger symptom onset compared to female ε4 carriers and males regardless of their APOE-ε4 status, suggesting a complex age and sex dependent relationship between APOE-ε4 and AD24. EOnonAD patients are less likely to be APOE-ε4 carriers, with a low, 14%, prevalence of the gene11,25.
This study aims to examine the effects of sex on gray matter atrophy, amyloid PET and tau PET burden and further stratify these effects by APOE-ε4 carrier status in the LEADS cohort. Given the limited literature on sex differences in EOAD and EOnonAD these analyses will fill a valuable gap in the literature. We hypothesized that EOAD females would have greater atrophy, amyloid PET, and tau PET burden than EOAD males. We also suspected that we might observe nonAD-like patterns of pathology in our EOnonAD group due to the multiple etiologies contributing to this disease state. Furthermore, we conducted an exploratory analysis in which we investigated how APOE-ε4 carrier status within each sex impacted EOAD and EOnonAD pathologic burden.
2. METHODS
2.1. Participants and Data collection
The current study included 230 EOAD, 70 EOnonAD, and 77 CN participants from the Longitudinal Early-Onset Alzheimer’s Disease Study (LEADS) with data from their baseline visit. When the study was conducted, 377 participants from LEADS had all necessary baseline imaging data needed for analysis which included T1 structural MRI, Florbetaben PET (FBB PET; amyloid), and Flortaucipir PET (FTP PET; tau) data. Due to batch processing APOE genotype data was only available for a subset of participants (204 EOAD, 67 EOnonAD, 74 CN).
LEADS is an ongoing longitudinal multi-site study with the primary goals of advancing knowledge of mechanisms and heterogeneity, and capturing disease progression through collection of clinical, genetic and biomarker data26. The study collects baseline and longitudinal clinical, cognitive, genetic, and neuroimaging and fluid biomarker data. Neuroimaging data collection includes various MRI sequences, FBB PET, FTP PET, and FDG PET (EOnonAD and CN groups only).
The framework, methodology, exclusion and inclusion criteria, and clinical and biomarker assessments of LEADS have been previously published26. Briefly, all participants are between 40–64 years old at the time of consent. Participants are either self-referred or referred from clinic and given the rarity of EOAD, site enrollment is not capped (nor is sex proportion). EOAD and EOnonAD participants meet the NIA-AA criteria for dementia or MCI and have a global Clinical Dementia Rating (CDR)27 score ≤ 1. CN participants have a global CDR = 0, a Mini-Mental State Examination (MMSE)28 score ≥ 24, and normal ranges on neuropsychological testing. Impaired individuals with genetic mutations in Amyloid Precursor Protein (APP), Presenilin-1 (PSEN1) or Presenilin-2 (PSEN2), Microtubule Associated Protein Tau (MAPT), Chromosome 9 Open Reading Frame 72 (C9ORF72), or Granulin Precursor aka Progranulin (GRN) were excluded, consistent with LEADS’ focus on sporadic early-onset dementia. To assign cognitively impaired participants to the EOAD or EOnonAD group, visual reads and global SUVR quantification of baseline amyloid PET scans were used. Certified physicians first performed visual reads blinded to clinical information and scan quantification. If the visual read and SUVR quantification were both Aβ-positive or both Aβ-negative, the participant was assigned to the EOAD or EOnonAD cohort, respectively. If the visual read and quantification were incongruent, a second “tie breaker” visual read was performed by an additional blinded reader for cohort assignment. The quantitative threshold used for amyloid PET positivity is a global SUVR≥1.18, extracted from a PET-only pipeline. The full details of all PET methods and thresholds are described elsewhere in this special issue29.
Informed consent was obtained from all participants in accordance with U.S. federal and local federations, the Declaration of Helsinki and the Indiana University Institutional Review Board.
2.2. Genotyping
National Alzheimer’s Coordinating Center, the Alzheimer’s Disease Center Network, National Centralized Repository for Alzheimer’s Disease and Related Dementias and Alzheimer’s Clinical Trials Consortium collaborate to track phenotypic data, imaging and biologic specimens, including genotypic data from LEADS participants30.
2.3. MRI and PET Data Acquisition and Processing
MRI and PET data acquisition details and any pre-processing steps are fully described in Apostolova et al.26. T1 structural MRI scans and fully pre-processed FBB PET and FTP PET scans were downloaded from LONI IDA31 in NifTI format. Fully pre-processed PET scans were already quality controlled, averaged, aligned to standard space, intensity-normalized and smoothed to standard resolution using ADNI procedures26,32. Volumetric measures for MRI and regional and composite standardized uptake value ratio (SUVR) values for amyloid and tau PET were downloaded from LONI, which were extracted using Freesurfer 7.1. Details on the methods to extract these values are described previously and elsewhere in this special issue26,29.
We processed the quality-controlled MRI scans using voxel-based morphometry (VBM) in Statistical Parametric Mapping version 12 (SPM12)33. The resulting gray matter maps were normalized and smoothed with a 10 mm Gaussian kernel which yielded gray matter density (GMD) images to be used in imaging analysis. The downloaded PET scans were co-registered to the corresponding MRI scan from the same visit, normalized to MNI space using transformation parameters from VBM MRI segmentation, and re-scaled to MRI-defined reference regions26,29 (amyloid PET to whole cerebellum and tau PET to inferior cerebellar gray matter) using SPM via MATLAB. Quality control was conducted on all final processed MRI and PET images.
2.4. Statistical analysis and Workflow
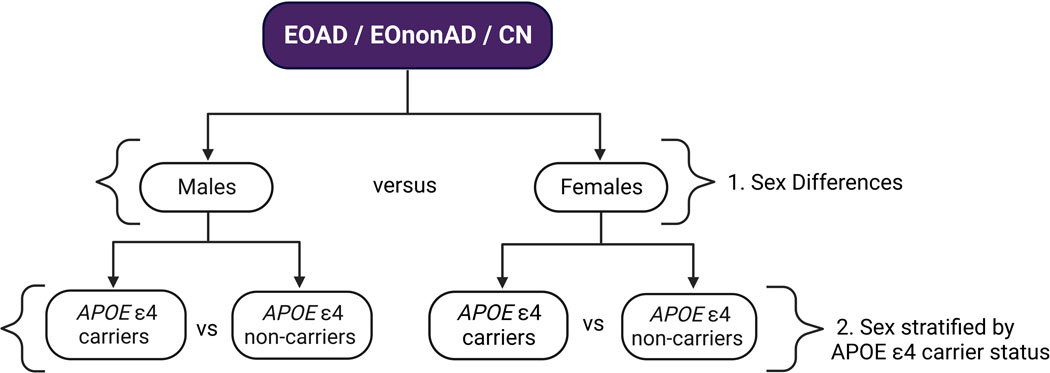
Our analyses included the full cohort and the subset of participants with available APOE genotype as described in section 2.1. First, we analyzed the effect of sex on imaging biomarkers within each diagnostic group. Next, we analyzed the effect of APOE-ε4 carrier status within males and females, separately, within each diagnostic group. See Figure 1 for analysis workflow.
Figure 1. Analysis Workflow.
A visual representation of the groups used for each analysis. 1) Males were compared to females in each diagnostic group for demographic, imaging biomarker and 3D analyses. 2) Male APOE-ε4 carriers were compared to male ε4 non-carriers and female APOE-ε4 carriers were compared to female ε4 non-carriers in each diagnostic group for demographic, imaging biomarker and 3D analyses.
2.4.1. Demographic and Imaging Biomarker Comparisons
Full cohort demographics were compared using analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables implemented in Statistical Package for Social Sciences (SPSS) version 28. Demographics and imaging biomarkers were compared using independent samples t tests for the sex differences and sex stratified by APOE-ε4 analyses. Box plot representations of means for the imaging biomarkers were produced in R Studio. We used average hippocampal volume as our MRI measure which was corrected for intracranial volume (ICV). For amyloid PET, we used a composite neocortical SUVR value representing global amyloid uptake. For tau PET, we used the MetaROI34 and composite Braak stage35 SUVR values (Braak 1/2, Braak 3/4, Braak 5/6).
2.4.2. 3D Comparisons
For analysis in imaging space, voxel-wise multiple linear regression models were employed in SPM 12 to produce whole brain 3D statistical brain maps of GMD, amyloid PET burden, and tau PET burden within each diagnostic group. Part one of our analyses used sex as the predictor (male vs. female). The second part of our analyses used APOE-ε4 carrier status (ε4+ vs. ε4-) as the predictor within each sex group. All models included age and education as covariates, and GMD models also included ICV as a covariate.
We set our voxel-wise significance threshold at p<0.01 (uncorrected) to visualize results and applied family-wise error (FWE) correction for multiple comparisons at the cluster level. Only clusters surviving a pFWE < 0.05 with a minimum cluster size (k) equal to the smallest significant cluster size were visualized. Cluster-corrected statistical maps were saved as NifTI files and rendered in Surf Ice 36 producing 3D brain maps.
3. RESULTS
3.1. Sample Characteristics
3.1.1. LEADS Cohort Overview
Demographic and baseline cognitive data for EOAD, EOnonAD, and CN groups are shown in Table 1. The CN group was younger than the EOAD and EOnonAD groups (p = .006). The CN group was also the most educated compared to EOAD and EOnonAD (p <.001). Predictably, the CN group had the highest MMSE score, followed by EOnonAD and lastly EOAD (p < .001).
Table 1. Sample Overview.
Demographic comparisons between EOAD, EOnonAD, and CN cohorts.
| EOAD | EOnonAD | CN | ||
|---|---|---|---|---|
| N | 230 | 70 | 77 | p-value |
| Age, years, mean (SD) | 59.1 (4.2) | 58.3 (6.2) | 56.5 (6.1) | 0.006 |
| Sex, % female | 54% | 36% | 68% | <0.001 |
| Race, % White | 94% | 89% | 75% | <0.001 |
| Years of education, mean (SD) | 15.5 (2.4) | 15.6 (2.5) | 16.7 (2.2) | <0.001 |
| APOE-ε4+ (%) | 54% | 42% | 43% | 0.114 |
| MMSE, mean (SD) | 21.6 (5.2) | 25.6 (4.2) | 29.3 (0.8) | <0.001 |
Sample Overview. Demographic comparisons between EOAD, EOnonAD, and CN groups.
While all groups were predominantly White non-Latino/a, the racial make-up of our cohort was significantly different across groups (p < .001). The CN and EOAD groups had larger proportions of females (68% and 54% respectively) compared to EOnonAD (36% female) (p < .001).
As expected, EOAD had the highest proportion of APOE-ε4 carriers, but the difference was not significant (CN 43%, EOAD 54%, and EOnonAD 42%; p = .114).
3.1.2. Demographic Differences by Sex
The results examining demographic differences by sex in each diagnostic group are shown in Table 2. There were no significant differences in age between males and females in either of the CN, EOAD, or EOnonAD groups. CN males had significantly more education than CN females (p = 0.04). There were no significant differences in years of education between males and females for EOnonAD or EOAD. Males and females in the CN and EOAD groups showed no significant differences on the MMSE, while EOnonAD males outperformed EOnonAD females on MMSE (p = .02). APOE-ε4 carrier status did not differ between males and females in any diagnostic group. Percentages of ε4 carriers in males and females in each diagnostic group are shown in Table 2.
Table 2. Sex Differences.
Demographic and imaging biomarker comparisons between EOAD, EOnonAD, and CN cohorts split by sex. Whole FBB SUVR = global amyloid uptake; FTP MetaROI SUVR, Braak 12, Braak 34, Braak 56 = tau uptake.
| EOAD | EOnonAD | CN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | p-value | Males | Females | p-value | Males | Females | p-value | |
| N | 105 | 125 | 45 | 25 | 25 | 52 | |||
| Age, years, mean (SD) | 59.1 (4.3) |
59.2 (4.1) |
0.9 | 58.3 (5.8) |
58.4 (6.8) |
0.9 | 55.6 (6.6) | 56.9 (5.8) |
0.4 |
| Education, years, mean (SD) | 15.3 (2.2) | 15.7 (2.5) |
0.2 | 15.9 (2.7) |
15.0 (2.1) |
0.1 | 17.4 (2.3) | 16.3 (2.1) |
0.04 |
| MMSE (SD) | 22.0 (5.4) | 21.3 (5.0) |
0.3 | 26.4 (2.4) |
24.0 (6.0) |
0.02 | 29.4 (0.7) | 29.3 (0.8) |
0.6 |
| Race, % White | 93% | 95% | 0.01 | 84% | 96% | 0.6 | 76% | 75% | 0.4 |
| APOE-ε4+ (%) | 57% | 51% | 0.4 | 40% | 46% | 0.6 | 33% | 48% | 0.2 |
| Whole FBB SUVR, mean, (SD) | 1.53 (0.2) | 1.61 (0.1) |
<0.001 | 1.00 (0.06) |
1.02 (0.07) |
0.3 | 1.03 (0.1) | 1.03 (0.06) | 0.8 |
| FTP MetaROI SUVR, mean (SD) | 2.00 (0.5) | 2.20 (0.4) |
<0.001 | 1.19 (0.2) |
1.27 (0.4) |
0.3 | 1.20 (0.2) | 1.14 (0.05) |
0.1 |
| BRAAK 12 SUVR, mean (SD) | 1.51 (0.2) | 1.56 (0.2) |
0.1 | 1.17 (0.1) |
1.19 (0.2) |
0.6 | 1.20 (0.2) | 1.14 (0.08) |
0.03 |
| BRAAK 34 SUVR, mean (SD) | 1.80 (0.4) | 1.95 (0.4) |
0.002 | 1.15 (0.2) |
1.21 (0.3) |
0.3 | 1.16 (0.2) | 1.11 (0.05) |
0.08 |
| BRAAK56 SUVR, mean (SD) | 1.75 (0.4) | 1.91 (0.4) |
0.006 | 1.07 (0.2) |
1.14 (0.3) |
0.2 | 1.08 (0.08) |
1.08 (0.05) |
0.8 |
| Hippocampal Vol, cm3, mean (SD) | 2.29 (0.3) | 2.39 (0.3) |
0.01 | 2.48 (0.4) |
2.64 (0.3) |
0.09 | 2.79 (0.3) | 2.77 (0.2) |
0.8 |
3.1.3. Sex Stratified by APOE-ε4 Carrier Status Demographics
The demographic results examining sex stratified by APOE-ε4 carrier status in each diagnostic group are shown in Table 3. There were no significant differences in age or education between female ε4 carriers and female ε4 non-carriers in the EOAD group. There were also no significant differences in age, education, or MMSE between male ε4 carriers and male ε4 non-carriers in the EOAD group. EOAD female carriers had a significantly higher MMSE score than EOAD female non-carriers (p = .01). Carrier and non-carrier differences by sex were not seen in either CN or EOnonAD for age, education, and MMSE.
Table 3.
Sex stratified by APOE-ε4 carrier status. Demographic and imaging biomarker comparisons in EOAD, EOnonAD, and CN cohorts split by sex and further stratified by APOE-ε4 carrier status. (A) EOAD male APOE-ε4 carriers vs. male non-carriers and EOAD female APOE-ε4 carriers vs. female non-carriers. (B) EOnonAD male APOE-ε4 carriers vs. male non-carriers and EOnonAD female APOE-ε4 carriers vs. female non-carriers. (C) CN male APOE-ε4 carriers vs. male non-carriers and CN female APOE-ε4 carriers vs. female non-carriers. Whole FBB SUVR = global amyloid uptake; FTP MetaROI SUVR, Braak 12, Braak 34, Braak 56 = tau uptake.
| (A)EOAD | ||||||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| APOE-ε4+ Carrier | Non-carrier | p-value | APOE-ε4+ Carrier | Non-carrier | p-value | |
| N | 56 | 54 | 54 | 40 | ||
| Age, years, mean (SD) | 62.8 (4.5) | 61.8 (3.8) | 0.3 | 62.3 (4.0) | 61.5 (4.6) | 0.4 |
| Education, years, mean (SD) | 15.5 (2.6) | 15.6 (2.5) | 0.9 | 15.1 (2.3) | 15.5 (2.2) | 0.3 |
| MMSE (SD) | 22.8 (4.5) | 20.4 (5.1) | 0.01 | 22.2 (0.5) | 21.5 (5.6) | 0.5 |
| APOE-ε4+ (%) | 51% | 57% | ||||
| Whole FBB SUVR, mean, (SD) | 1.56 (0.2) | 1.65 (0.1) | <0.001 | 1.54 (0.2) | 1.53 (0.2) | 0.8 |
| FTP MetaROI SUVR, mean (SD) | 2.13 (0.4) | 2.24 (0.4) | 0.2 | 1.93 (0.5) | 2.05 (0.4) | 0.2 |
| BRAAK 12 SUVR, mean (SD) | 1.62 (0.2) | 1.51 (0.2) | 0.004 | 1.51 (0.2) | 1.48 (0.2) | 0.5 |
| BRAAK 34 SUVR, mean (SD) | 1.91 (0.4) | 1.98 (0.3) | 0.3 | 1.76 (0.4) | 1.82 (0.3) | 0.4 |
| BRAAK 56 SUVR, mean (SD) | 1.83 (0.5) | 1.98 (0.4) | 0.09 | 1.70 (0.5) | 1.81 (0.4) | 0.2 |
| Hippocampal Vol, cm 3 , mean (SD) | 2.34 (0.3) | 2.42 (0.3) | 0.09 | 2.26 (0.3) | 2.34 (0.2) | 0.2 |
| (B)EOnonAD | ||||||
| Female | Male | |||||
| APOE-ε4+ Carrier | Non-carrier | p-value | APOE-ε4+ Carrier | Non-carrier | p-value | |
| N | 11 | 13 | 17 | 26 | ||
| Age, years, mean (SD) | 61.0 (7.6) | 61.0 (5.6) | 0.9 | 62.6 (5.8) | 61.0 (6.0) | 0.4 |
| Education, years, mean (SD) | 14.4 (2.0) | 15.4 (2.2) | 0.3 | 16.2 (2.6) | 15.7 (2.9) | 0.6 |
| MMSE (SD) | 24.8 (6.4) | 23.4 (6.1) | 0.6 | 26.6 (2.7) | 26.6 (2.1) | 0.1 |
| APOE-ε4+ (%) | 46% | 40% | ||||
| Whole FBB SUVR, mean, (SD) | 1.04 (0.09) | 1.00 (0.05) | 0.2 | 1.01 (0.08) | 1.00 (0.06) | 0.3 |
| FTP MetaROI SUVR, mean (SD) | 1.27 (0.4) | 1.28 (0.4) | 0.1 | 1.24 (0.3) | 1.15 (0.07) | 0.2 |
| BRAAK 12 SUVR, mean (SD) | 1.18 (0.2) | 1.19 (0.2) | 0.9 | 1.19 (0.2) | 1.15 (0.1) | 0.4 |
| BRAAK 34 SUVR, mean (SD) | 1.22 (0.4) | 1.21 (0.3) | 0.1 | 1.19 (0.3) | 1.12 (0.06) | 0.2 |
| BRAAK 56 SUVR, mean (SD) | 1.15 (0.3) | 1.13 (0.2) | 0.8 | 1.11 (0.3) | 1.04 (0.05) | 0.2 |
| Hippocampal Vol, cm 3 , mean (SD) | 2.65 (0.2) | 2.64 (0.4) | 0.9 | 2.41 (0.5) | 2.52 (0.3) | 0.4 |
| (C)CN | ||||||
| Female | Male | |||||
| APOE-ε4+ Carrier | Non-carrier | p-value | APOE-ε4+ Carrier | Non-carrier | p-value | |
| N | 24 | 26 | 8 | 16 | ||
| Age, years, mean (SD) | 59.7 (5.2) | 60.8 (6.1) | 0.5 | 58.0 (6.8) | 60.1 (6.2) | 0.5 |
| Education, years, mean (SD) | 16.3 (1.8) | 16.4 (2.5) | 0.9 | 17.3 (3.0) | 17.5 (2.0) | 0.8 |
| MMSE, mean (SD) | 29.4 (0.7) | 29.2 (0.9) | 0.3 | 29.4 (0.7) | 29.3 (0.7) | 0.8 |
| APOE-ε4+ (%) | 48% | 33% | ||||
| Whole FBB SUVR, mean, (SD) | 1.04 (0.07) | 1.02 (0.06) | 0.2 | 1.08 (0.2) | 1.01 (0.1) | 0.2 |
| FTP MetaROI SUVR, mean (SD) | 1.14 (0.06) | 1.15 (0.05) | 0.4 | 1.31 (0.4) | 1.15 (0.06) | 0.2 |
| BRAAK 12 SUVR, mean (SD) | 1.13 (0.1) | 1.15 (0.07) | 0.4 | 1.29 (0.2) | 1.16 (0.07) | 0.06 |
| BRAAK 34 SUVR, mean (SD) | 1.11 (0.06) | 1.12 (0.04) | 0.5 | 1.24 (0.3) | 1.13 (0.06) | 0.2 |
| BRAAK 56 SUVR, mean (SD) | 1.07 (0.05) | 1.08 (0.04) | 0.5 | 1.11 (0.1) | 1.06 (0.06) | 0.3 |
| Hippocampal Vol, cm 3 , mean (SD) | 2.78 (0.2) | 2.77 (0.3) | 0.8 | 2.75 (0.3) | 2.81 (0.3) | 0.6 |
3.2. Imaging biomarker and 3D comparisons
3.2.1. Sex Differences
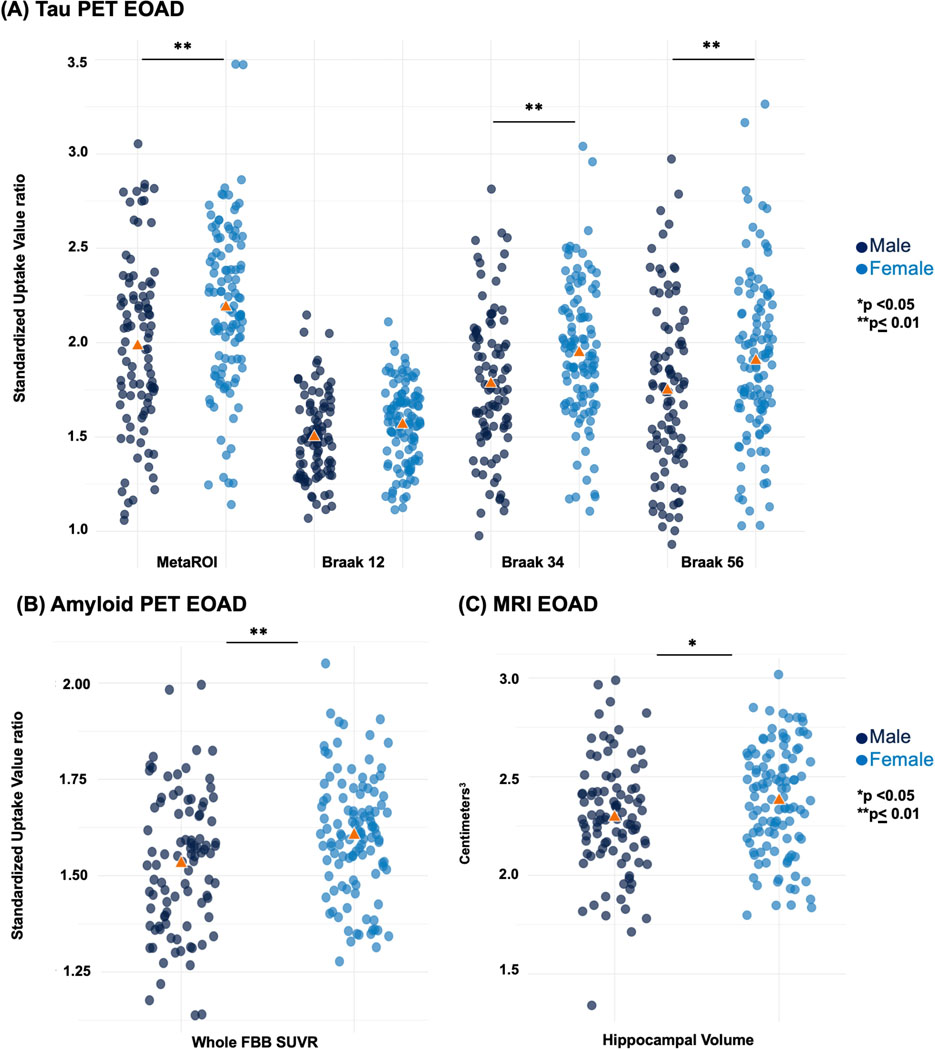
Imaging biomarker comparisons between males and females are shown in Table 2 for all groups and displayed as box plots in Figure 2 for the EOAD group. In the EOAD group, females had significantly greater tau uptake in the metaROI (p < .001), as well as Braak 3/4 (p = .002) and Braak 5/6 (p = .006) and higher mean global amyloid uptake, compared to EOAD males (p < .001). Interestingly, EOAD females also had larger mean hippocampal volume than EOAD males (p = .01). Sex-based differences were also seen in the CN group (Supplementary Figure 1). CN males had a greater mean tau SUVR in Braak 1/2 (p = .03). There were no significant sex-based differences in the EOnonAD group (Supplementary Figure 1).
Figure 2. Box plots - Sex differences.
Imaging biomarker measures comparing EOAD males and females: (A) tau uptake measures, (B) global amyloid uptake, (C) hippocampal volume.
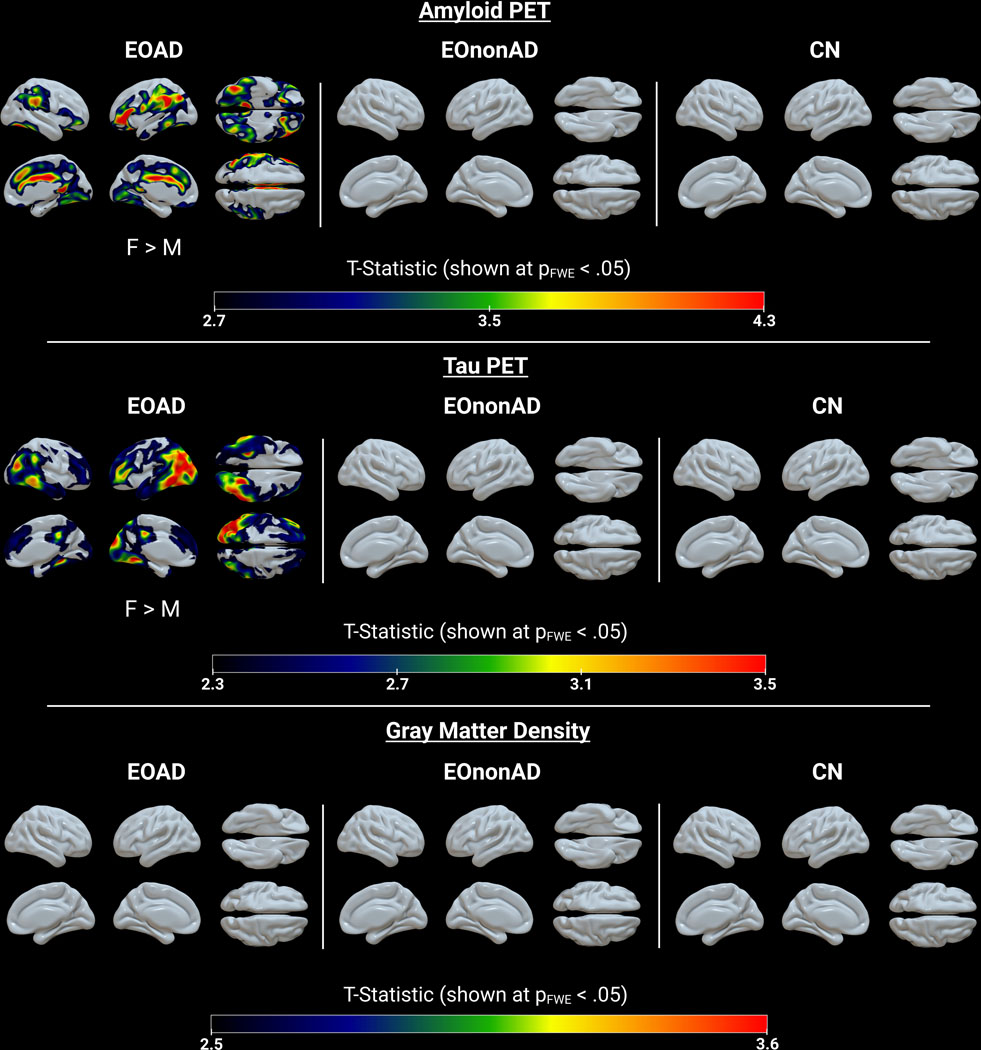
The FWE cluster-level corrected whole brain maps from the voxel-wise analysis comparing male and female participants within CN, EOAD, and EOnonAD are shown in Figure 3. Overall, EOAD females had greater amyloid and tau burden than EOAD males. For amyloid PET, females showed greater uptake than males in the frontal, inferior parietal, and inferior temporal cortices and the superior frontal, cingulate, and lingual gyri, bilaterally. For tau PET, females showed greater uptake than males primarily in the left temporoparietooccipital and the left middle and inferior temporal cortices. No significant differences between males and females were seen in the CN and EOnonAD groups for amyloid or tau burden. There were also no significant differences between males and females for GMD in CN, EOAD, or EOnonAD.
Figure 3. 3D comparisons – Sex Differences.
Voxel-wise multiple linear regression statistical maps showing males vs. females within CN, EOAD, and EOnonAD for amyloid PET, tau PET and GMD. All GMD models were controlled for ICV. FWE: family-wise error.
3.2.2. Sex stratified by APOE-ε4 carrier status
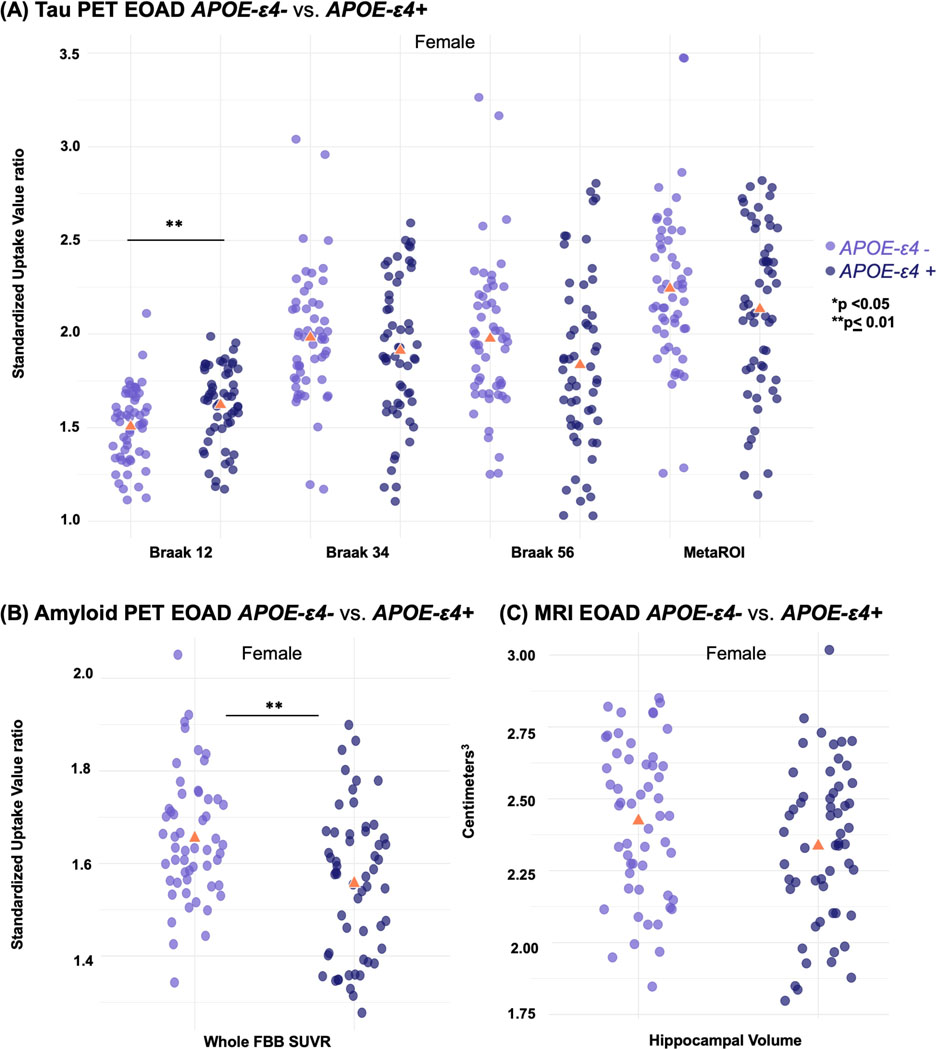
Imaging biomarker comparisons for sex stratified by APOE-ε4 carrier status are shown in Table 3 for all groups and displayed as box plots in Figure 4 for the EOAD group. In the EOAD group, female ε4 carriers had a higher mean tau SUVR in Braak 1/2 (p = .004) than female ε4 non-carriers; however, it was the female ε4 non-carriers with greater global amyloid uptake (p < .001). No significant differences were found between male ε4 carriers and male ε4 non-carriers in the EOAD group nor between carriers and non-carriers of any sex in the EOnonAD and CN groups (Supplementary Figures 2 & 3).
Figure 4. Box plots - Sex stratified by APOE-ε4 carrier status.
Imaging biomarker measures comparing EOAD female APOE-ε4 carriers (+) vs. female non-carriers (−): (A) tau uptake measures, (B) global amyloid uptake, (C) hippocampal volume.
The FWE cluster-level corrected whole brain maps from the voxel-wise analysis comparing female ε4 carriers with female ε4 non-carriers within CN, EOAD, and EOnonAD are shown in Figure 5. There were no significant differences between male ε4 carriers and male ε4 non-carriers for GMD, amyloid or tau uptake in any of the diagnostic groups (not shown; all clusters, pFWE > .05).
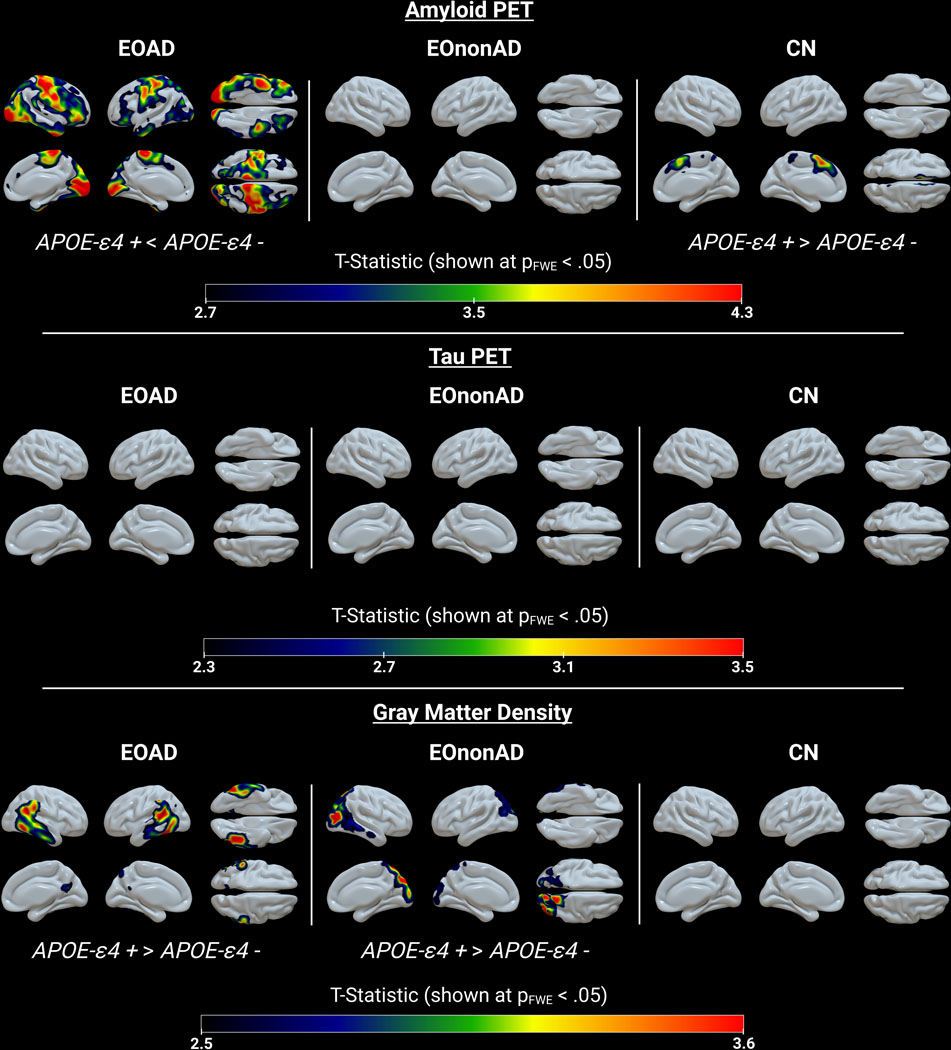
Figure 5. 3D Comparisons – Sex stratified by APOE-ε4 carrier status.
Voxel-wise multiple linear regression statistical maps showing female APOE-ε4 carriers (+) vs female non-carriers (−) within CN, EOAD, and EOnonAD for amyloid PET, tau PET and GMD. All GMD models were controlled for ICV. FWE: family-wise error.
CN female ε4 carriers had greater amyloid uptake than CN female ε4 non-carriers in the superior and medial frontal cortices. In contrast, EOAD female ε4 non-carriers had greater amyloid uptake compared to EOAD female ε4 carriers in the paracentral, frontal, and occipital cortices and the lingual gyrus and temporal pole. This pattern was present bilaterally but more pronounced on the right. EOAD female ε4 non-carriers also had greater gray matter atrophy than EOAD female ε4 carriers in the lateral temporal, temporoparietooccipital and inferior parietal cortices, bilaterally.
EOnonAD female ε4 non-carriers had greater gray matter atrophy lateralized to the right occipital cortex compared to EOnonAD female ε4 carriers. There were no significant differences between female ε4 carriers and female ε4 non-carriers for GMD in CN, for amyloid uptake in EOnonAD, or for tau uptake in EOAD, EOnonAD or CN.
4. DISCUSSION
Results of our imaging biomarker comparisons demonstrated that both sex and APOE-ε4 carrier status impact atrophy, amyloid PET, and tau PET burden in EOAD, EOnonAD, and CN individuals.
4.1. Sex Differences
Greater amyloid and tau PET burden have been previously reported in female LOAD patients compared to male patients37. As hypothesized, we found that female patients with EOAD have greater amyloid PET and tau PET burden compared to male patients. Examining the regional distribution of amyloid and tau burden in EOAD females, we observed greater amyloid uptake in frontal, parietal and temporal areas, and greater tau uptake in temporoparietooccipital areas. Consistent with previous findings38, MMSE scores were similar between females and males despite greater pathological burden in females, suggesting that female AD patients may handle greater pathology burden and show some degree of cognitive resiliency. Thus, sex may serve as a moderator of the association between amyloid and tau burden and cognitive function39.
We also found that EOAD females had significantly larger mean hippocampal volume than EOAD males which was seen in MCI and AD patients in prior work40, suggesting that females could be less atrophied. However, we did not observe differences in GMD between males and females in our voxel-wise regression, similar to previous cross-sectional results41,42. While no cross-sectional differences were seen, Lee et al.41, did observe longitudinal changes in cortical thickness between males and females with AD, showing females had more rapid decline than males. This highlights the importance of examining longitudinal changes between sexes within the LEADS cohort in future studies.
The role of sex hormones, such as estrogen, and menopause are important considerations in this age group given their proximity to menopause. Differences in estrogen levels across the lifespan have been linked to risk of dementia. Prevalence of dementia in surgically induced menopause versus natural menopause, and the association between number of pregnancies and rates of AD suggest low estrogen is associated with increased vulnerability to development of AD in female patients37,43–50. Examining the influence of sex hormone fluctuations on cognitive function and neuroimaging biomarkers throughout the lifecycle is an important future direction for understanding sex-linked vulnerabilities to dementia. This may be particularly fruitful in EO cohorts whose younger age offer a unique and essential perspective by including pre and perimenopausal women.
4.2. Sex stratified by APOE-ε4 carrier status
The most novel aspect of this paper is the further division of sex-split groups to account for APOE-ε4 carrier status. Novel, exploratory analyses splitting each diagnosis group by sex and then by APOE-ε4 carrier status showed important effects of ε4 carrier status. Across all diagnostic groups, APOE-ε4 carrier status was the most impactful in females.
Our CN group had a 43% APOE-ε4 carrier rate and while this is not significantly higher than those of the EOAD and EOnonAD groups, it is notably higher compared to the global average proportion of APOE-ε4 carriers among CN individuals in the population (23.9%)51. This high carrier rate can be attributed to the CN group being comprised of highly motivated volunteers who may have familial connections to AD.
CN female APOE-ε4 carriers had more amyloid PET burden in the medial frontal cortices compared to female non-carriers, but no differences were found for tau burden. Amyloid deposition and APOE have been shown to interact in cognitively normal individuals, leading to a cascade of decline52,53. Mormino and Papp54 have suggested that in older clinically normal individuals, increased amyloid burden may represent a disease precursor and link to expedient decline. These findings and our data in conjunction with evidence that AD disproportionately affects female patients a nearly 2:1 rate over male patients, suggest a sex-driven uptake disparity in amyloid. Mosconi et al.55, reported that cognitively normal perimenopausal and postmenopausal women exhibit increased amyloid deposition, hypometabolism, and reduced gray and white matter volume in regions vulnerable to AD when compared with cognitively normal premenopausal women and age-matched men. Further, postmenopausal APOE-ε4 carriers had greater amyloid deposition relative to the other groups55.
Similar to LOAD, EOAD female APOE-ε4 carriers showed greater tau burden in the medial temporal lobe56–58. The present study found EOAD female APOE-ε4 carriers to have greater tau uptake in entorhinal and parahippocampal cortices (Braak 1/2) than female non-carriers. These findings reinforce that the APOE-ε4 genotype facilitates a predisposition to vulnerability in the medial temporal areas 57–59.
However, EOAD female APOE-ε4 non-carriers showed greater amyloid uptake than female carriers in the late Braak regions corresponding to the primary sensorimotor and visual cortices and greater gray matter atrophy, particularly in the lateral temporal, temporoparietooccipital and inferior parietal cortices. These findings suggest that there are yet uncovered risk factors that drive greater amyloid and neurodegenerative pathology burden in female ε4 non-carriers. Less well-understood risk variants and genetic modifiers in EOAD that may be worth investigating for sex-based differences include PRNP p.MM129V, SORL1, and CCL11 p.A23T, and MAPT60–62. Consideration of these and other genetic drivers are essential in illuminating a more comprehensive understanding of AD disease processes and presentations and paving a path for greater sex and gender equity in the research, diagnosis, and precision-medicine-based treatment of EOAD.
Our results also yielded a significant difference between female ε4 carriers and female ε4 non-carriers in the EOnonAD group. EOnonAD female ε4 non-carriers presented with greater gray matter atrophy than ε4 carriers, primarily in the right occipital cortex. Given the heterogeneity of EOnonAD, these results may reinforce or, at least, not contradict, previous assertions that EOnonAD neurodegeneration is not driven by the APOE pathway, but rather a unique spectrum of etiologies that differ from AD63.
4.3. Strengths and Limitations
Several strengths and limitations of our study should be noted. We expanded on the first part of our analysis examining sex differences by also exploring the impact of APOE-ε4 carrier status on gray matter atrophy, amyloid PET, and tau PET burden. By first splitting each diagnostic group by males and females, then further dividing each sex by APOE-ε4 carrier status, we were able to create a novel sequence of analyses and generate a new set of data.
One of the limitations of our study is the cross-sectional nature of our analyses. Longitudinal analyses are needed to assess atrophy and amyloid and tau uptake overtime; however, LEADS is a longitudinal study and future efforts will be focused on longitudinal changes. Another limitation is that our study focused on the effects of sex and APOE-ε4 carrier status on neuroimaging biomarkers, so we did not include analysis of cognitive measures beyond MMSE. This limits our interpretation of the cognitive profiles in our cohort but is an opportunity for future research to explore cognitive resiliency and variation between sexes. It is also important to acknowledge that our analyses do not account for menopausal status for female participants. These data are currently being collected to include in future analyses examining the effects of menopause on cognitive function and neuroimaging biomarkers once the full study cohort is enrolled. Additionally, LEADS employs relatively strict inclusion and exclusion criteria which limit the representation of the cohort to all Alzheimer’s and dementia patients. Furthermore, insufficient post-mortem data has been collected thus far in LEADS as it is an ongoing study, so pathological verification of AD and non-AD pathologies are lacking. In terms of diversity, equity and inclusion, the LEADS population is largely middle-aged, highly educated, and primarily consists of non-Latino/a White adults. As such, our findings do not generalize well to the AD population at large. Though our current sample lacks diversity, the LEADS study recently added a diversity recruitment supplement to promote community outreach of diverse populations. Replication of our findings in a more diverse group will be essential in ensuring greater inclusivity and applicability of our research.
4.4. Future Directions
These analyses are preliminary, and we plan to continue building on this work. Longitudinal follow-up analyses of atrophy, amyloid PET, and tau PET will provide important insight on outcomes among diagnostic groups. Additionally, we plan to explore cognitive resiliency between sexes by including more neuropsychiatric variables, further dividing groups by amnestic and non-amnestic presentation, and adding fluid biomarkers to our analysis. Other risk factors (such as obesity, hypertension, diabetes, depression etc.) that could be contributing to sex differences in neuroimaging biomarkers should also be included in future work. There is also an opportunity to compare the characteristics of our EOAD population in LEADS to other AD populations with known genetic risk such as dominantly inherited Alzheimer’s disease from the Dominantly Inherited Alzheimer Network Observational Study.
4.5. Conclusion
Despite our limitations, our results show that APOE-ε4 and sex play a role in AD pathology in EOAD and suggest that the effects may be at least in part different from those observed in LOAD studies. Uncovering the main components of disease heterogeneity by examining the differential effects of sex, age, and genetic risk brings us closer to characterizing these understudied disease states and implementing clinical interventions.
Supplementary Material
HIGHLIGHTS.
Novel analysis examining the effects of biological sex and apolipoprotein E ε4 (APOE-ε4) carrier-status on neuroimaging biomarkers among early-onset Alzheimer’s disease (EOAD), early-onset non-AD (EOnonAD), and cognitively normal (CN) participants.
Female sex is associated with greater pathology burden in the EOAD cohort compared to male sex.
The effect of APOE-ε4 carrier status on pathology burden was the most impactful in females across all cohorts.
ACKNOWLEDGEMENTS
We would like to thank all members of the LEADS Consortium. We would also like to thank the Apostolova Lab for their continued guidance and support. We would like to express special thanks to LEADS participants and their family members and friends and to study staff and administrative personnel, without whose effort and time this research would not have been possible. All figures were created with BioRender.com.
FUNDING SOURCES
This study is generously supported by R56 AG057195, U01AG6057195, U24AG021886, Alzheimer’s Association AARG-22-926940, Alzheimer’s Association LEADS GENETICS‐19‐639372, Alzheimer’s Association LDRFP-21-818464, Alzheimer’s Association LDRFP-21-824473 and Alzheimer’s Association LDRFP-21-828356. NACC is funded by the NIA (U24 AG072122). NACC data are contributed by the following NIA-funded ADRCs: P30 AG010133, P30 AG062422, P30 AG066462, P30AG066507, P30 AG062421, P30 AG066506, P30AG072977, P30 AG066444, P30 AG066515, P30 AG062677, P30 AG072980, P30 AG072979, P30 AG066511.
Footnotes
CONFLICTS
J.L.D is an inventor on patents or patent applications of Eli Lilly and Company relating to the assays, methods, reagents and / or compositions of matter related to measurement of P-tau217. J.L.D. has served as a consultant for Abbvie, Genotix Biotechnologies Inc, Gates Ventures, Karuna Therapeutics, AlzPath Inc, Cognito Therapeutics, Inc., and received research support from ADx Neurosciences, Fujirebio, AlzPath Inc, Roche Diagnostics and Eli Lilly and Company in the past two years. J.L.D. has received speaker fees from Eli Lilly and Company.
L.I is currently a full-time employee of Eli Lilly and Company / Avid Radiopharmaceuticals and a minor shareholder of Eli Lilly and Company. His contribution to the work presented in this manuscript was performed while he was affiliated with the University of California San Francisco.
L.G.A has provided consultation to Eli Lilly, Biogen, Two Labs, FL Dept Health, Genentech, NIH Biobank, Eli Lilly, GE Healthcare, Eisai, Roche Diagnostics, and Alnylam. L.G.A receives the following research support: NIA U01 AG057195, NIA R01 AG057739, NIA P30 AG010133, Alzheimer Association LEADS GENETICS 19-639372, Alzheimer Association SG-23-1061716, Roche Diagnostics RD005665, AVID Pharmaceuticals, Life Molecular Imaging. L.G.A has received honoraria for participating in independent data safety monitoring boards and providing educational CME lectures and programs. L.G.A has stock in Cassava Sciences.
No other authors associated with this project have reported conflicts of interest that would impact these results.
CONSENT STATEMENT
All authors have read and provided consent to be associated with this manuscript.
REFERENCES
- 1.Apostolova LG. Alzheimer Disease . Continuum (Minneap Minn). Apr 2016;22(2 Dementia):419–34. doi: 10.1212/con.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Vlies AE, Koedam EL, Pijnenburg YA, Twisk JW, Scheltens P, van der Flier WM. Most rapid cognitive decline in APOE ɛ4 negative Alzheimer’s disease with early onset. Psychol Med. Nov 2009;39(11):1907–11. doi: 10.1017/s0033291709005492 [DOI] [PubMed] [Google Scholar]
- 3.Polsinelli AJ, Logan PE, Lane KA, et al. APOE ε4 carrier status and sex differentiate rates of cognitive decline in early- and late-onset Alzheimer’s disease. Alzheimers Dement. Nov 17 2022;doi: 10.1002/alz.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polsinelli AJ, Lane KA, Manchella MK, Logan PE, Gao S, Apostolova LG. APOE ε4 is associated with earlier symptom onset in LOAD but later symptom onset in EOAD. Alzheimers Dement. May 2023;19(5):2212–2217. doi: 10.1002/alz.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tort-Merino A, Falgas N, Allen IE, et al. Early-onset Alzheimer’s disease shows a distinct neuropsychological profile and more aggressive trajectories of cognitive decline than late-onset. Ann Clin Transl Neurol. Dec 2022;9(12):1962–1973. doi: 10.1002/acn3.51689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stage EC Jr., Svaldi D, Phillips M, et al. Neurodegenerative changes in early- and late-onset cognitive impairment with and without brain amyloidosis. Alzheimers Res Ther. Aug 5 2020;12(1):93. doi: 10.1186/s13195-020-00647-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon B, Shim YS, Park HK, Park SA, Choi SH, Yang DW. Predictive factors for disease progression in patients with early-onset Alzheimer’s disease. J Alzheimers Dis. 2016;49(1):85–91. doi: 10.3233/jad-150462 [DOI] [PubMed] [Google Scholar]
- 8.Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. Feb 2017;11(1):205–213. doi: 10.1007/s11682-016-9523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/clep.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contador J, Perez-Millan A, Guillen N, et al. Sex differences in early-onset Alzheimer’s disease. Eur J Neurol. Dec 2022;29(12):3623–3632. doi: 10.1111/ene.15531 [DOI] [PubMed] [Google Scholar]
- 11.Chételat G, Ossenkoppele R, Villemagne VL, et al. Atrophy, hypometabolism and clinical trajectories in patients with amyloid-negative Alzheimer’s disease. Brain. 2016;139(9):2528–2539. doi: 10.1093/brain/aww159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dani M, Brooks DJ, Edison P. Suspected non-Alzheimer’s pathology – Is it non-Alzheimer’s or non-amyloid? Ageing Research Reviews. 2017/07/01/ 2017;36:20–31. doi: 10.1016/j.arr.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Wisse LEM, de Flores R, Xie L, et al. Pathological drivers of neurodegeneration in suspected non-Alzheimer’s disease pathophysiology. Alzheimer’s Research & Therapy. 2021/05/14 2021;13(1):100. doi: 10.1186/s13195-021-00835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. Nov 5 2014;84(3):608–22. doi: 10.1016/j.neuron.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR Jr., Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. Jun 2012;71(6):765–75. doi: 10.1002/ana.22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. Nov 2014;71(11):1379–85. doi: 10.1001/jamaneurol.2014.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. Oct 22-29 1997;278(16):1349–56. [PubMed] [Google Scholar]
- 18.Sundermann EE, Tran M, Maki PM, Bondi MW. Sex differences in the association between apolipoprotein E epsilon4 allele and Alzheimer’s disease markers. Alzheimers Dement (Amst). 2018;10:438–447. doi: 10.1016/j.dadm.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolova LG, Risacher SL, Duran T, et al. Associations of the Top 20 Alzheimer Disease Risk Variants With Brain Amyloidosis. JAMA Neurol. Mar 1 2018;75(3):328–341. doi: 10.1001/jamaneurol.2017.4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017/09/01 2017;549(7673):523–527. doi: 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stage E, Risacher SL, Lane KA, et al. Association of the top 20 Alzheimer’s disease risk genes with [(18)F]flortaucipir PET. Alzheimers Dement (Amst). 2022;14(1):e12308. doi: 10.1002/dad2.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson Y, Gibbons L, Pritchard A, et al. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(1):60–6. doi: 10.1159/000097038 [DOI] [PubMed] [Google Scholar]
- 23.Smirnov DS, Galasko D, Hiniker A, Edland SD, Salmon DP. Age-at-Onset and APOE-Related Heterogeneity in Pathologically Confirmed Sporadic Alzheimer Disease. Neurology. May 4 2021;96(18):e2272–e2283. doi: 10.1212/wnl.0000000000011772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettens K, Sleegers K, Van Broeckhoven C. Genetic insights in Alzheimer’s disease. Lancet Neurol. Jan 2013;12(1):92–104. doi: 10.1016/s1474-4422(12)70259-4 [DOI] [PubMed] [Google Scholar]
- 25.Shimada H, Ataka S, Takeuchi J, et al. Pittsburgh compound B-negative dementia: a possibility of misdiagnosis of patients with non-alzheimer disease-type dementia as having AD. J Geriatr Psychiatry Neurol. Sep 2011;24(3):123–6. doi: 10.1177/0891988711409410 [DOI] [PubMed] [Google Scholar]
- 26.Apostolova LG, Aisen P, Eloyan A, et al. The Longitudinal Early-onset Alzheimer’s Disease Study (LEADS): Framework and methodology. Alzheimers Dement. Dec 2021;17(12):2043–2055. doi: 10.1002/alz.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris JC. The Clinical Dementia Rating (CDR). Current version and scoring rules. 1993;43(11):2412–2412-a. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 28.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. Sep 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- 29.Cho H, Mundada NS, Apostolova LG, al. e. Amyloid and Tau-PET in Early-onset AD: Baseline Data from the Longitudinal Early-onset Alzheimer’s Disease Study (LEADS). Alzheimer’s and Dementia. 2023;this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Alzheimer’s Coordinating Center. NACC Partnerships. Accessed May 23, 2023. https://naccdata.org/nacc-collaborations/partnerships [Google Scholar]
- 31.Laboratory of Neuro Imaging Image and Data Archive. Accessed May 23, 2023. https://ida.loni.usc.edu/ [Google Scholar]
- 32.Jagust WJ, Landau SM, Koeppe RA, et al. The Alzheimer’s Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimers Dement. Jul 2015;11(7):757–71. doi: 10.1016/j.jalz.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitwell JL. Voxel-Based Morphometry: An Automated Technique for Assessing Structural Changes in the Brain. The Journal of Neuroscience. 2009;29(31):9661–9664. doi: 10.1523/jneurosci.2160-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR Jr., Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimer’s & Dementia. 2017;13(3):205–216. doi: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [(18)F]-AV-1451 tau PET data. Data Brief. Dec 2017;15:648–657. doi: 10.1016/j.dib.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SurfIce. Accessed May 23, 2023. https://www.nitrc.org/projects/surfice/ [Google Scholar]
- 37.Buckley RF, O’Donnell A, McGrath ER, et al. Menopause Status Moderates Sex Differences in Tau Burden: A Framingham PET Study. Ann Neurol. Jul 2022;92(1):11–22. doi: 10.1002/ana.26382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards L, La Joie R, Iaccarino L, et al. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer’s continuum: greater tau-PET retention in females. Neurobiology of Aging. 2021/09/01/ 2021;105:86–98. doi: 10.1016/j.neurobiolaging.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liesinger AM, Graff-Radford NR, Duara R, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. Dec 2018;136(6):873–885. doi: 10.1007/s00401-018-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundermann EE, Biegon A, Rubin LH, et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology. 2016;86(15):1368–1376. doi: 10.1212/wnl.0000000000002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Cho H, Jeon S, et al. Sex-Related Reserve Hypothesis in Alzheimer’s Disease: Changes in Cortical Thickness with a Five-Year Longitudinal Follow-Up. Journal of Alzheimer’s Disease. 2018;65:641–649. doi: 10.3233/JAD-180049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo SW, Im K, Lee J-M, et al. Effects of demographic factors on cortical thickness in Alzheimer’s disease. Neurobiology of Aging. 2011/02/01/ 2011;32(2):200–209. doi: 10.1016/j.neurobiolaging.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 43.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. Apr 2011;32(4):604–13. doi: 10.1016/j.neurobiolaging.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ptok U, Barkow K, Heun R. Fertility and number of children in patients with Alzheimer’s disease. Arch Womens Ment Health. Oct 2002;5(2):83–6. doi: 10.1007/s00737-002-0142-6 [DOI] [PubMed] [Google Scholar]
- 45.McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci. Spring 2003;15(2):161–7. doi: 10.1176/jnp.15.2.161 [DOI] [PubMed] [Google Scholar]
- 46.Colucci M, Cammarata S, Assini A, et al. The number of pregnancies is a risk factor for Alzheimer’s disease. Eur J Neurol. Dec 2006;13(12):1374–7. doi: 10.1111/j.1468-1331.2006.01520.x [DOI] [PubMed] [Google Scholar]
- 47.Beeri MS, Rapp M, Schmeidler J, et al. Number of children is associated with neuropathology of Alzheimer’s disease in women. Neurobiol Aging. Aug 2009;30(8):1184–91. doi: 10.1016/j.neurobiolaging.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. Mar 16 2011;1379:188–98. doi: 10.1016/j.brainres.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bove R, Secor E, Chibnik LB, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. Jan 21 2014;82(3):222–9. doi: 10.1212/wnl.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imtiaz B, Tuppurainen M, Tiihonen M, et al. Oophorectomy, hysterectomy, and risk of Alzheimer’s disease: a nationwide case-control study. J Alzheimers Dis. 2014;42(2):575–81. doi: 10.3233/jad-140336 [DOI] [PubMed] [Google Scholar]
- 51.Wang Y-Y, Ge Y-J, Tan C-C, Cao X-P, Tan L, Xu W. The Proportion of APOE4 Carriers Among Non-Demented Individuals: A Pooled Analysis of 389,000 Community-Dwellers. Journal of Alzheimer’s Disease. 2021;81:1331–1339. doi: 10.3233/JAD-201606 [DOI] [PubMed] [Google Scholar]
- 52.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. Jan 2010;67(1):122–31. doi: 10.1002/ana.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. May 19 2015;313(19):1924–38. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mormino EC, Papp KV. Amyloid Accumulation and Cognitive Decline in Clinically Normal Older Individuals: Implications for Aging and Early Alzheimer’s Disease. J Alzheimers Dis. 2018;64(s1):S633–s646. doi: 10.3233/jad-179928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology. Sep 26 2017;89(13):1382–1390. doi: 10.1212/wnl.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emrani S, Arain HA, DeMarshall C, Nuriel T. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: a systematic review. Alzheimer’s Research & Therapy. 2020/11/04 2020;12(1):141. doi: 10.1186/s13195-020-00712-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. Apr 2014;75(4):563–73. doi: 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. Dec 2018;136(6):887–900. doi: 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Flier WM, Pijnenburg YAL, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE ɛ4 allele. The Lancet Neurology. 2011/03/01/ 2011;10(3):280–288. doi: 10.1016/S1474-4422(10)70306-9 [DOI] [PubMed] [Google Scholar]
- 60.Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer’s & Dementia. 2016/06/01/ 2016;12(6):733–748. doi: 10.1016/j.jalz.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 61.Lalli MA, Bettcher BM, Arcila ML, et al. Whole-genome sequencing suggests a chemokine gene cluster that modifies age at onset in familial Alzheimer’s disease. Molecular Psychiatry. 2015/11/01 2015;20(11):1294–1300. doi: 10.1038/mp.2015.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. Sep 2011;10(9):785–96. doi: 10.1016/s1474-4422(11)70156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hohman TJ, Dumitrescu L, Oksol A, Wagener M, Gifford KA, Jefferson AL. APOE allele frequencies in suspected non-amyloid pathophysiology (SNAP) and the prodromal stages of Alzheimer’s Disease. PLoS One. 2017;12(11):e0188501. doi: 10.1371/journal.pone.0188501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.