Abstract
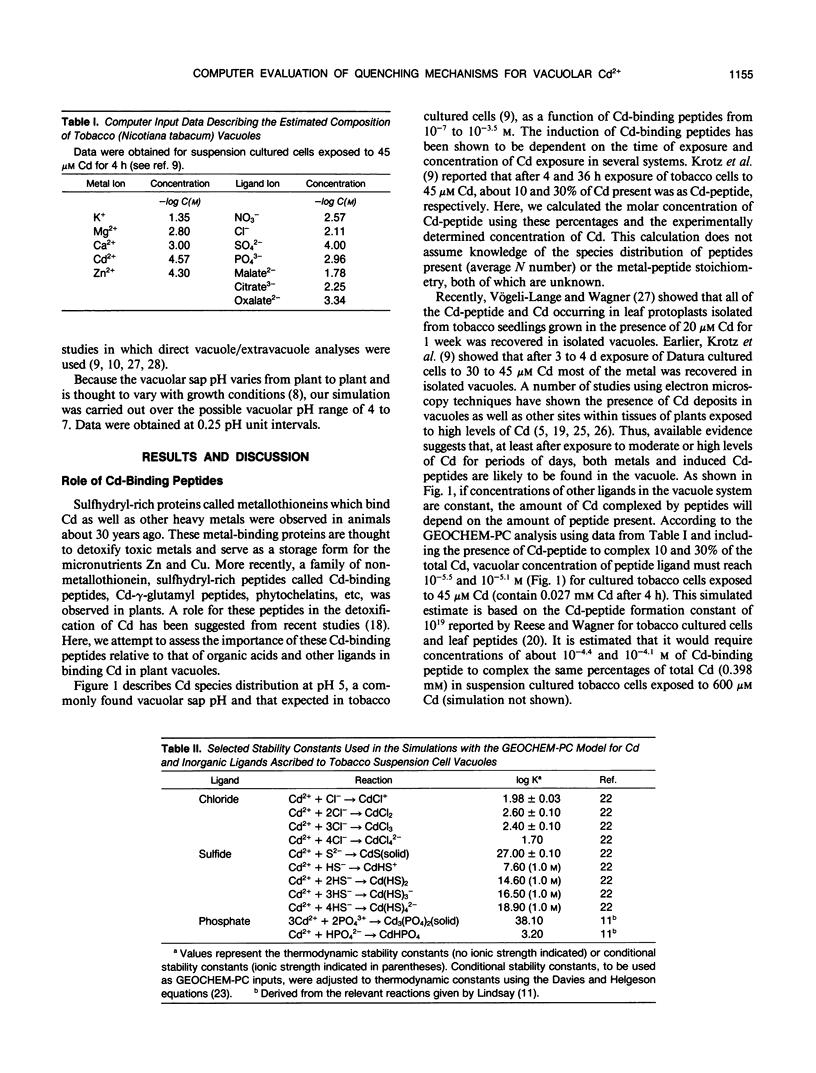
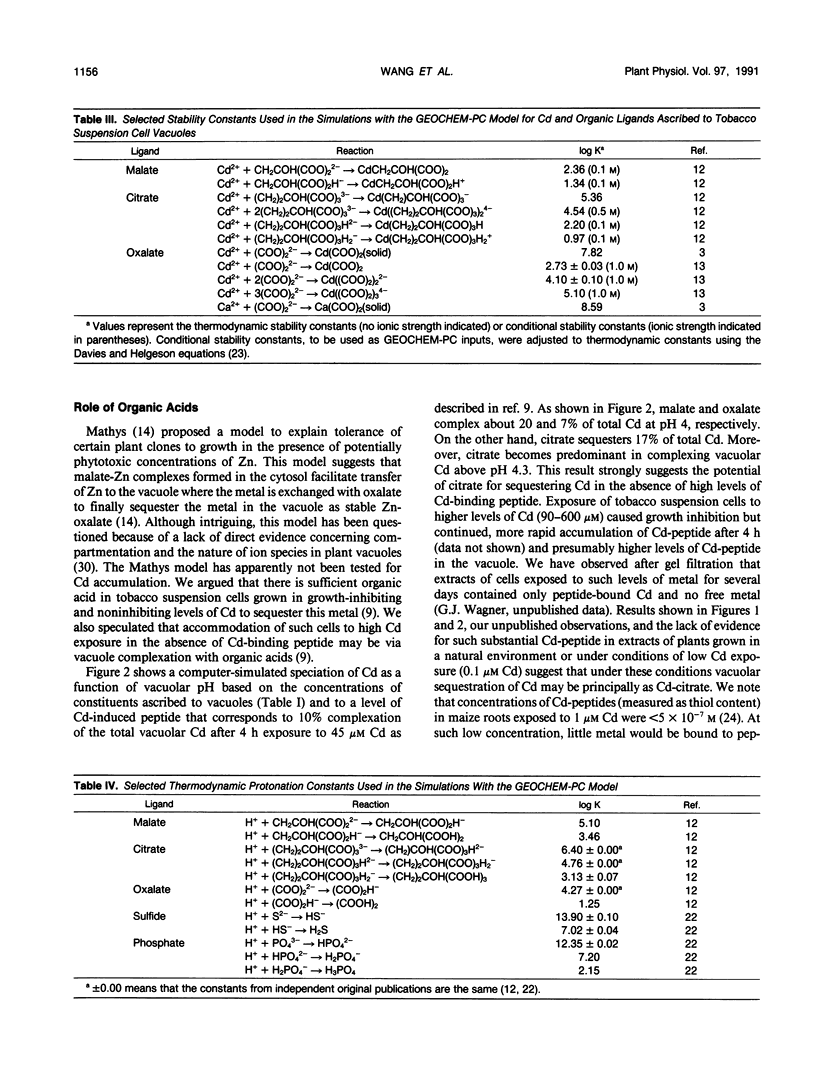
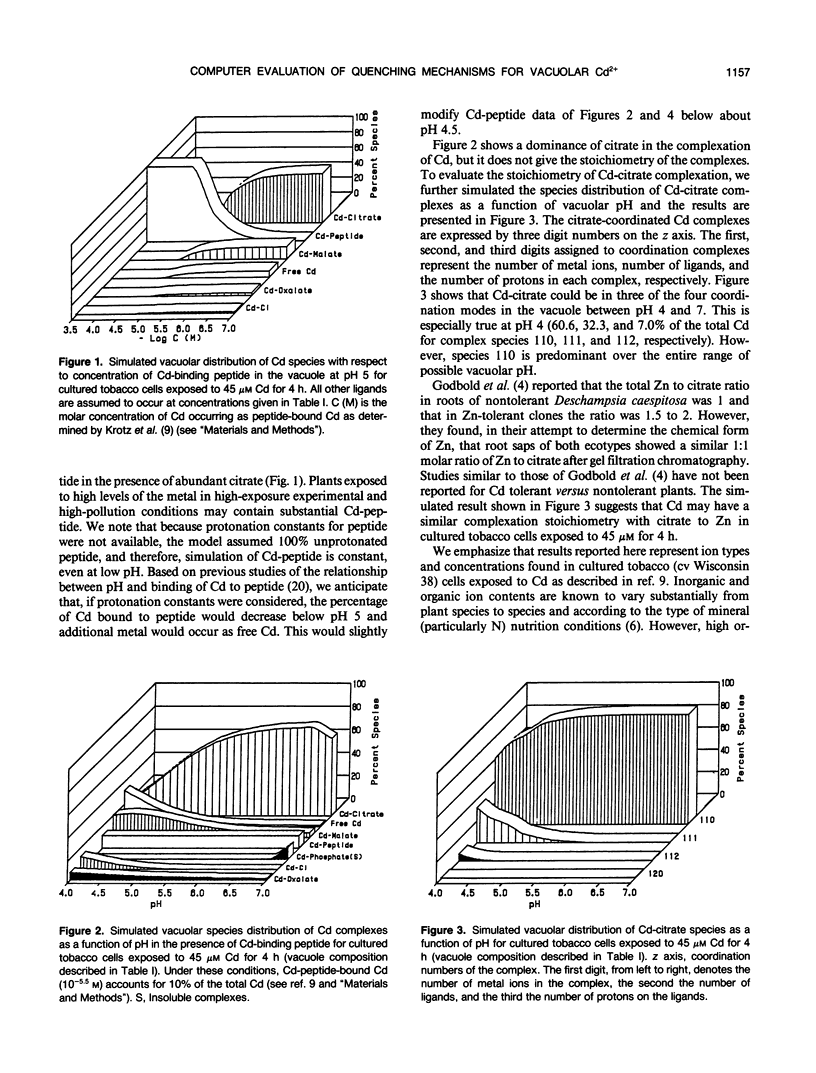
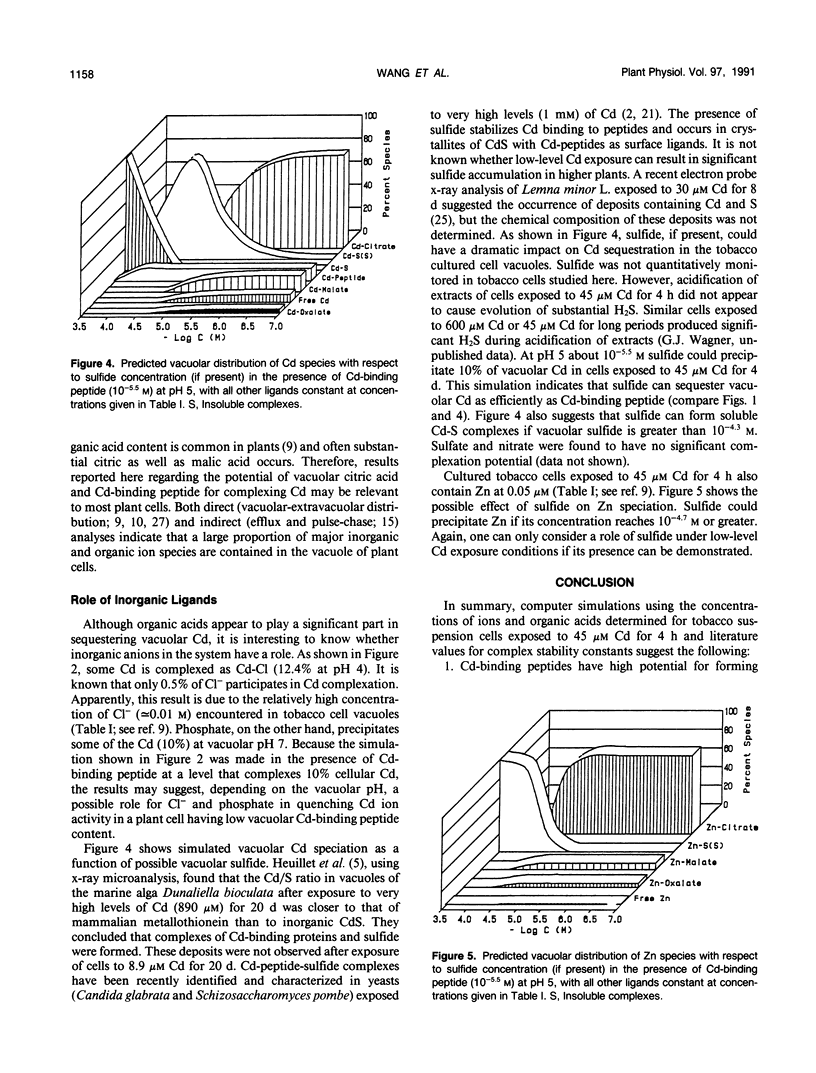
Various mechanisms have been suggested for the quenching of Cd ion activity in plant vacuoles. These include solution complexation with organic acids and sulfhydryl-containing peptides and precipitation as sulfides. Because direct experimental support for these mechanisms is lacking and difficult to obtain, we have used a computer model to evaluate the quenching role of possible organic and inorganic ligands of tobacco cultured cells exposed to Cd. Results of this thermodynamic evaluation, which assumes that a chemical equilibrium state is met in the vacuole, support the conclusion that sulfhydryl-containing peptides and certain organic acids may form soluble Cd complexes. Although complexation of malate and oxalate with Cd is predicted to be less significant, citrate in the concentration range encountered in the tobacco cultured cell vacuoles has high potential for forming soluble complexes with Cd over the entire possible vacuolar pH range, especially 4.3 to 7.0, even in the presence of low levels of Cd-binding peptides. In addition, results show that inorganic chloride, sulfide (if present), and phosphate may also act to sequester Cd ion activity in the vacuole by forming soluble Cd-Cl and insoluble CdS and Cd-phosphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Krotz R. M., Evangelou B. P., Wagner G. J. Relationships between Cadmium, Zinc, Cd-Peptide, and Organic Acid in Tobacco Suspension Cells. Plant Physiol. 1989 Oct;91(2):780–787. doi: 10.1104/pp.91.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Rauser W. E. Phytochelatins. Annu Rev Biochem. 1990;59:61–86. doi: 10.1146/annurev.bi.59.070190.000425. [DOI] [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Properties of tobacco (Nicotiana tabacum) cadmium-binding peptide(s). Unique non-metallothionein cadmium ligands. Biochem J. 1987 Feb 1;241(3):641–647. doi: 10.1042/bj2410641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. N., Winge D. R. Sulfide stabilization of the cadmium-gamma-glutamyl peptide complex of Schizosaccharomyces pombe. J Biol Chem. 1988 Sep 15;263(26):12832–12835. [PubMed] [Google Scholar]
- Vögeli-Lange R., Wagner G. J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves : implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990 Apr;92(4):1086–1093. doi: 10.1104/pp.92.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. C. Metal Complexation in Xylem Fluid : II. THEORETICAL EQUILIBRIUM MODEL AND COMPUTATIONAL COMPUTER PROGRAM. Plant Physiol. 1981 Feb;67(2):301–310. doi: 10.1104/pp.67.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


