Abstract
Pain is a main symptom in inflammation, and inflammation induces pain via inflammatory mediators acting on nociceptive neurons. Macrophages and microglia are distinct cell types, representing immune cells and glial cells, respectively, but they share similar roles in pain regulation. Macrophages are key regulators of inflammation and pain. Macrophage polarization plays different roles in inducing and resolving pain. Notably, macrophage polarization and phagocytosis can be induced by specialized pro-resolution mediators (SPMs). SPMs also potently inhibit inflammatory and neuropathic pain via immunomodulation and neuromodulation. In this review, we discuss macrophage signaling involved in pain induction and resolution, as well as in maintaining physiological pain. Microglia are macrophage-like cells in the central nervous system (CNS) and drive neuroinflammation and pathological pain in various inflammatory and neurological disorders. Microglia-produced inflammatory cytokines can potently regulate excitatory and inhibitory synaptic transmission as neuromodulators. We also highlight sex differences in macrophage and microglial signaling in inflammatory and neuropathic pain. Thus, targeting macrophage and microglial signaling in distinct locations via pharmacological approaches, including immunotherapies, and non-pharmacological approaches will help to control chronic inflammation and chronic pain.
Keywords: inflammation, macrophages, microglia, neuroinflammation, pain
Introduction
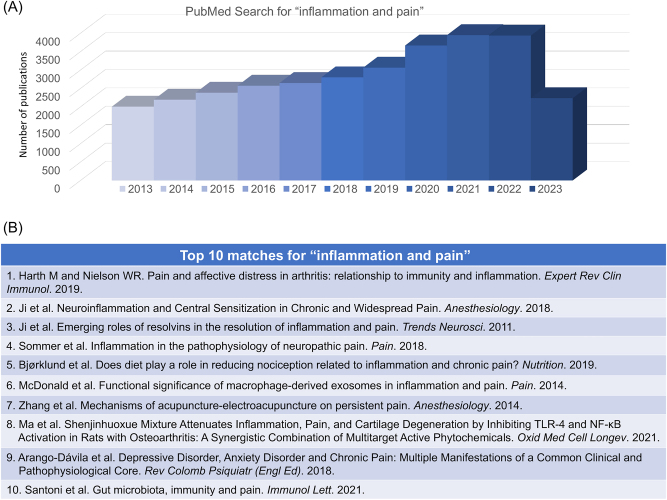
Inflammation is a critical process in biological host defense against infection and injury and enables host survival, although it comes at the cost of a transient functional loss or disturbance in the affected tissue [1]. The inflammatory process involves the recruitment of various immune cells, including neutrophils, macrophages, and lymphocytes, to the site of injury or infection. These immune cells release chemical mediators such as cytokines, chemokines, and prostaglandins, which regulate the inflammatory responses and activate and sensitize nearby sensory nerves (nociceptive fibers/nociceptors) to cause pain. Pain is one of the five main symptoms of inflammation, which also includes edema, warmth, redness, and loss of function [2]. We conducted a PubMed search for key words “inflammation and pain” on July 15, 2023, which resulted in 47,675 publications. We observed a trend of increase in the related publications in the past decade from 2013 to 2023 (Figure 1A). We also included the top 10 papers with the best match (Figure 1B).
Figure 1:
PubMed search for “inflammation and pain”, conducted on July 15, 2023. (A) Distribution of the related papers in the past 10 years (2013–2023). This search shows a total of 47,675 publications. (B) Top 10 matches: 1) Harth and Neilson [3]; 2) Ji et al. [4]; 3) Ji et al. [5]; 4) Sommer et al. [6]; 5) Bjørklund et al. [7]; 6) McDonald et al. [8]; 7) Zhang et al. [9]; 8) Ma et al. [10]; 9) Arango-Dávila et al. [11]; 10) Santoni et al. [12].
Acute pain acts as a protective mechanism that helps animals and humans detect and avoid dangerous or potentially dangerous stimuli [13]. In contrast, chronic pain is a pathological condition with no such benefit. Pain is triggered by the activation of a subset of primary sensory neurons called nociceptors. Nociceptors are a diverse group of neurons that can be characterized by factors such as their myelination level, soma size, electrophysiological properties, and molecular markers [14–18]. Primary nociceptors have cell bodies in the dorsal root ganglia (DRG) and trigeminal ganglia (TG) and send sensory projections to various peripheral tissues like the skin, muscles, joints, and internal organs. The peripheral axons and nerve fibers of nociceptors interact with non-neuronal cells (e.g., keratinocytes and unmyelinated Schwann cells) [19–24], and resident immune cells such as macrophages, mast cells, and dendritic cells [25–29].
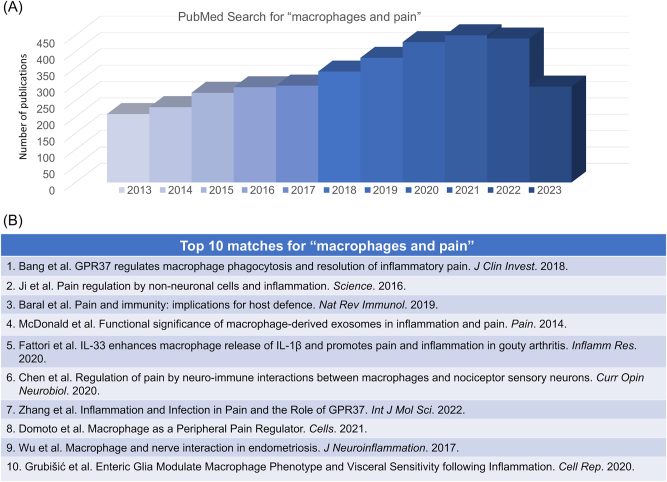
The immune system and nervous system are critical to and corporate with each other during the host defense and nociceptive responses [30–32]. This neuro-immune interaction involves multiple pathways and cell types, including neurons, glial cells, immune cells, and other non-neuronal cells [25]. Macrophages are the most studied immune cells in pain research, and a PubMed search for “Macrophages and pain” on July 15 of 2023 resulted in 5,153 citations (Figure 2A and B). Macrophages originate from hematopoietic myeloid progenitors in the bone marrow. These immune cells can infiltrate peripheral tissues such as skin, joint, nerve, and DRG tissue, as well as spinal cord tissue after peripheral tissue injuries including nerve injury. Macrophages can release cytokines and chemokines to directly activate nerve fibers. They release additional inflammatory mediators such as ROS, causing nerve damage that contributes to chronic pain. The interaction between the nervous system and the immune system is bidirectional, with each system influencing the other. This complex interplay is critical for the development and maintenance of pain sensitization [25]. Targeting this neuro-immune interaction may be a promising approach for the development of new pain treatments.
Figure 2:
PubMed search for “macrophages and pain”, conducted on July 15, 2023. (A) Distribution of the related papers in the past 10 years (2013–2023). This search results in a total of 5,153 publications. (B) Top 10 matches. 1) Bang et al. [33]; 2) Ji et al. [25]; 3) Baral et al. [34]; 4) McDonald et al. [8]; 5) Fattori et al. [35]; 6) Chen et al. [36]; 7) Zhang et al. [37]; 8) Domoto et al. [38]; 9) Wu et al. [39]; 10) Grubišić et al. [40].
Microglia are the resident immune cells of the central nervous system (CNS), including the brain and spinal cord. They originate from yolk sac progenitor cells during early embryonic development and migrate to the CNS, where they differentiate into microglia. Unlike macrophages, which continuously differentiate from bone marrow-derived precursors, microglia have a unique developmental origin, which contributes to their specialized functions within the CNS. Importantly, microglia interact with the central axons and terminals of nociceptors as well as postsynaptic neurons in the spinal cord and brainstem [41]. Accumulating evidence suggests that microglia have both detrimental and beneficial roles in pain [42]. Microglia play an important role in inducing and maintaining neuroinflammation in the CNS, driving chronic pain and its comorbidities (e.g., depression, anxiety, and cognitive decline) [4, 5, 43, 44]. In this review article, we will discuss the role of macrophages and microglia in inflammation, neuroinflammation, and pain with special focus on neuro-immune crosstalk.
Role of macrophages in inflammation and pain
Macrophages are a type of immune cell that play a key role in the body’s defense against pathogens and foreign substances. As an important part of the innate immune system, macrophages are found in almost all tissues throughout the body. Macrophages are derived from monocytes, which make up 2 %–8 % of white blood cells and circulate in the bloodstream. Macrophages are classified according to their location or by their phenotype and function. Resident macrophages are found in different tissues including DRG and bone. For example, osteoclasts are resident macrophages in bone tissue and contribute to bone cancer pain [45, 46]. In response to invading pathogens and tissue injury, the infiltrating macrophages migrate and infiltrate into the damaged tissue and co-ordinate with the tissue-resident macrophages to regulate inflammatory responses. Macrophages have three major roles during host-defense: phagocytosis, cytokine production and antigen presentation. Macrophages recognize foreign invaders through their surface receptors, such as toll-like receptor (TLRs). Polarized macrophages have different functional states in which different cytokines are involved. For example, M1-like macrophages can intensify pain through the release of pro-inflammatory cytokines and chemokines. M2-like macrophages can reduce pain and repair damaged tissue through the release of anti-inflammatory cytokines and growth factors. For antigen presenting, macrophages are particularly effective at presenting antigens to T cells due to their high expression of MHC class II molecules and their ability to secrete cytokines and other immune-modulatory molecules that can activate and regulate T cell responses. Additionally, macrophages can interact with other immune cells, such as dendritic cells and B cells, to coordinate and regulate the immune response during the inflammation [2].
Primary sensory neurons express various molecular sensors such as transient receptor potential channels (e.g., TRPA1, TRPV1), Piezo channels (e.g., Piezo 1), voltage-gated sodium channels (e.g., Nav1.7–1.9), G-protein coupled receptors (GPCRs), and TLRs. These sensors can detect physical stimuli, cytokines and chemokines, and danger signals [47–51]. Emerging studies have demonstrated that there is a bidirectional interaction between primary sensory neurons and macrophages. Not only do macrophages secrete cytokines to act on neurons, they also receive “messages” (e.g., neuropeptides and chemokines) from primary afferents. Macrophages play a chief role in the pathogenesis of pain and can both induce and relieve pain via distinct inflammatory mediators [36].
Induction of pain by macrophages
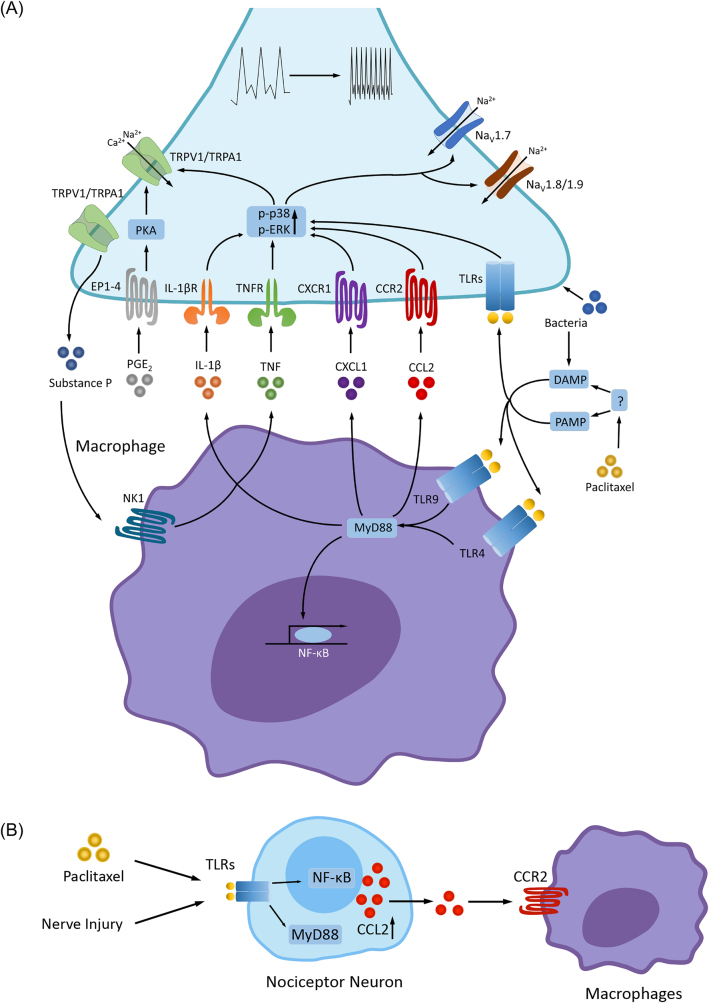
Upon stimulation after tissue injury or infection, macrophages produce and secrete inflammatory cytokines, growth factors, and lipids which directly stimulate nociceptors terminals and generate pain (Figure 3). Substance P exerts a significant impact on macrophages and can be synthesized when nociceptors, particularly C-fibers, are activated. Substance P stimulates the production of proinflammatory cytokines like TNF-α, IL-1β, and IL-6 by activating the ERK-p38 MAPK-mediated NF-κB pathway through NK-1 on the cell surface. However, emerging evidence suggests that substance P also promotes M2-like macrophages for tissue repair after brain injury [36, 52]. In particular, the accumulation of IL-1β-expressing macrophages is a crucial factor in the pathogenesis of pain. Prostaglandin E2 (PGE2), which enhances inflammatory pain, can activate neuronal EP1-EP4 receptors. Furthermore, PGE2 can stimulate the NF-κB pathway via PKA and PKC in DRG neurons, resulting in persistent hyperalgesia [53, 54]. ROS generated by macrophages can also activate TRPA1 in nociceptors, inducing pain. Additionally, complement fragment C5a can induce thermal hyperalgesia by cooperating with nerve growth factor (NGF) and TRPV1 [55, 56]. It was also found that elevated serum IL-20 levels are associated with severe sensory pain in cancer patients after paclitaxel treatment, and moreover, blocking IL-20 with a neutralizing antibody prevented the development of CIPN and dampened inflammatory responses, including macrophage infiltration and cytokine expression in mouse DRG [57].
Figure 3:
Macrophages and induction of pain. (A) In macrophages, activation of TLRs (e.g., TLR4 and TLR9) by PAMPs and DAMPs [50] increases the synthesis and release of inflammatory cytokines and chemokines (TNF, IL-1β, IL-17, CCL2, CXCL1) and lipid mediators (e.g., PGE2) via MyD88 and NF-κB pathways. PAMP and DAMP can be induced by bacterial and viral infections, tissues injury, or chemotherapy (e.g., paclitaxel). These inflammatory mediators act on their respective receptors (e.g., cytokine/chemokine receptors and EP1-EP4 receptors for PGE2) that are expressed on nociceptors [53, 54, 58, 59], leading to the receptor-mediated signaling transduction through phosphorylation of MAPKs (p-p38 and p-ERK) and activation of protein kinase A (PKA) [5, 60, 61]. Upon activation, these kinases then enhance the activities of ion channels (e.g., TRPA1/TRPV1 and voltage-gated sodium channels NaV1.7, NaV1.8 and NaV1.9) via posttranslational modulations, leading to increased sensitivity and excitability of nociceptors (peripheral sensitization) and increased pain sensitivity [62–64]. Bacteria is also known to produce pain via specific receptors and ion channels expressed by nociceptors [65]. Furthermore, activation of TRPA1/V1 in nociceptors releases substance P, which binds NK1 receptor on macrophages to release of TNF and IL-1β. In addition, nociceptor neurons express TLRs (e.g., TLR4 and TLR7), and activation of nociceptor TLRs by PAMP and DAMP (e.g., bacteria) can elicit pain. PAMP and DAMP can be indirectly generated by induction of chemotherapy. (B) After nerve injury and chemotherapy, nociceptor neurons produce CCL2 via activation of TLRs, MyD88 and NF-κB. CCL2 activates macrophages via CCR2 and induce macrophage infiltration in the DRG and nerve tissues [66–68]. CCL2, chemokine ligand 2; CCR2, chemokine ligand 2 receptor; DAMP, damage-associated molecular pattern molecules; MAPK, mitogen-activated protein kinase; PAMP, pathogen-associated molecular pattern molecules; TLRs, toll-like receptors. Reproduced from Chen et al. [36] with CCC permission.
Not only do nociceptors “listen to” macrophages via cytokines, they also “talk to” macrophages via signal molecules such as neuropeptides and chemokines. C-C motif chemokine ligand 2 (CCL2), also known as monocyte chemoattractant protein 1 (MCP1), is a pro-inflammatory chemokine produced by monocytic cells, including macrophages. Nerve injury was shown to upregulate CCL2 levels in DRG neurons, which in turn promotes peripheral sensitization by activation of CCR2 receptors in nociceptive neurons [58, 66]. Furthermore, CCL2 release from the central terminals of nociceptors can directly modulate synaptic plasticity in postsynaptic neurons that express CCR2 [69]. Subsequently, after chemotherapy treatment, CCL2 can be produced by nociceptors and can regulate peripheral macrophage activation in DRG, ultimately leading to neuropathic pain [66]. This process is initiated by the activation of TLR signaling through the myeloid differentiation factor 88 (MyD88) pathway in nociceptors [67, 70].
Aside from cytokines, chemokines, and neuropeptides, microRNAs (miRNA) also play a role in mediating interactions between macrophages and nociceptors. MiRNAs are small single-stranded non-coding functional RNAs and function in RNA silencing and regulation of protein expression. Since miRNAs can be secreted extracellularly in painful disease conditions, extracellular miRNAs can stimulate intracellular communication and produce physiological effects [71, 72]. Specifically, the release of miR-21-5p by nociceptors can regulate gene expression in macrophages, promoting a pro-inflammatory phenotype that can contribute to the development of neuropathic pain through TGF-β signaling [73, 74].
Resolution of pain by macrophages
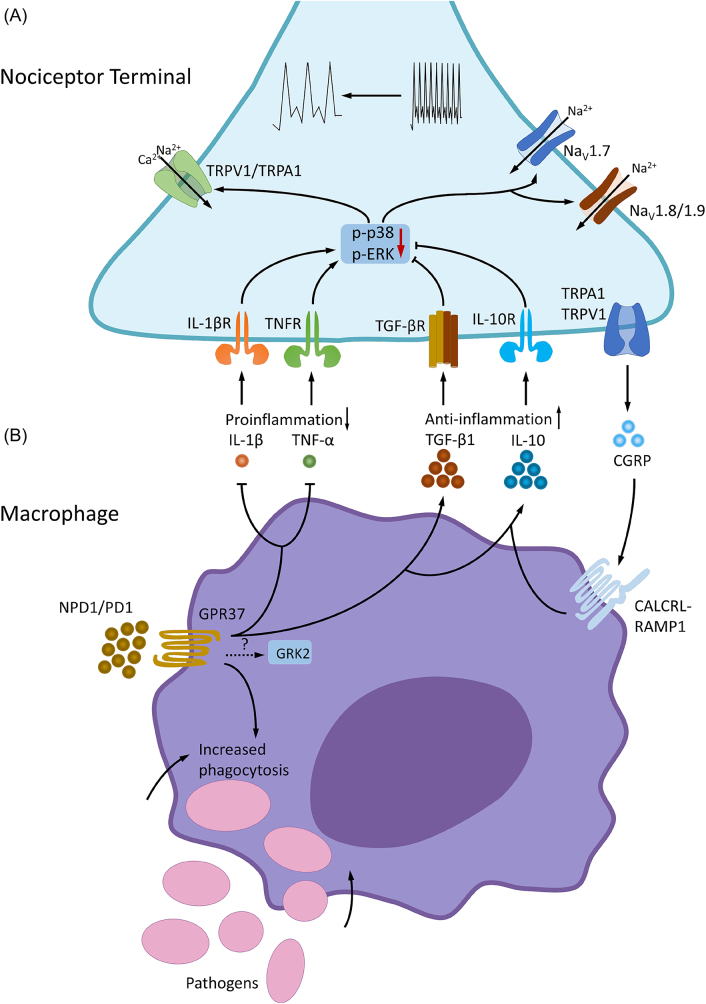
Accumulating evidence also points to an active role of macrophage in the resolution of pain after inflammation and infection [33, 75] (Figure 4). Macrophage polarization has been strongly implicated in inflammatory disease modulation [76]. M1-like macrophages are pro-inflammatory and produce pro-inflammatory cytokines, such as IL-1β and TNF-α, which promote pain. In contrast, M2-like macrophages are immunosuppressive and secrete anti-inflammatory cytokines (IL-10, TGF-β1), and neuropeptide (CGRP) to promote tissue repair and facilitate resolution of pain [36, 77]. Notably, macrophages could have various phenotypes and polarization states, in addition to M1 and M2 like states [78]. Macrophages also produce specialized pro-resolving mediators (SPMs) derived from omega-3 unsaturated fatty acids, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) for the resolution of inflammation and pain [79–82]. For example, macrophage proresolving mediator maresin 1 (MaR1) was found to stimulate macrophage phagocytosis, tissue regeneration, and control inflammatory and neuropathic pain [79, 83–85].
Figure 4:
Macrophages and resolution of pain. (A) Regulation of pain resolution via macrophage-nociceptor interactions. Macrophages express GPR37, a newly identified SPM receptor. Activation of macrophage GPR37 by NPD1 promotes phenotypic switch from M1-like macrophages to M2-like macrophages, which contribute to the resolution of inflammation and pain via increased release of IL-10 and TGF-β and decreased release of IL-1β and TNF. IL-10 and TGF-β can act on their respective receptors on nociceptors to inhibit peripheral sensitization [86, 87]. Activation of GPR37 also induces phagocytosis of pathogens (e.g., zymosan) and apoptotic neutrophils, which is a critical step for the resolution of inflammation and pain. IL-10 production in macrophages is also regulated by GRK2 [88], but the connection between GPR37 and GRK2 is still unknown. In addition, CGRP released from nociceptor neurons can regulate the anti-inflammatory function via its receptor complex CALCRL-RAMP1 on macrophages to enhance the expression of IL-10. CALCRL, calcitonin receptor-like receptor; NPD1, neuroprotectin D1; RAMP1, receptor activity-modifying protein 1; SPM, specialized pro-resolving mediator. Reproduced from Ref. [36] with permission. (B) Neuroprotectin D1/protectin D1 (NPD1/PD1), a SPM derived from DHA, induces phagocytosis of zymosan particles of peritoneal macrophages via GPR37. Note that NPD1-induced phagocytosis is diminished in macrophages from Gpr37 knockout mice. Reproduced from Chen et al. [36] with CCC permission.
Calcitonin gene-related peptide (CGRP), which has two isoforms, α-CGRP and β-CGRP, is a neuropeptide that can be produced by nociceptors. CGRP exhibits anti-inflammatory properties by reducing cytokine synthesis, lowering ROS production levels and enhancing IL-10 levels in myeloid cells. In macrophages, CGRP interacts with a receptor complex composed of the G-protein coupled receptors calcitonin receptor-like receptor (CALCRL) and receptor activity-modifying protein 1 (RAMP1). A deficiency in the RAMP1 component of the CGRP receptor leads to diminished immunosuppression due to decreased IL-10 levels [36, 89].
Macrophages express the orphan receptor GPR37, which is activated by neuroprotectin D1 (NPD1), a SPM derived from DHA omega-3 unsaturated fatty acids. GPR37 activation in macrophages contributes to resolving inflammatory pain and infection-induced pain through phagocytosis of apoptotic neutrophils and macrophage polarization [37]. GPR37 regulates macrophage polarization and promote M2 phenotype by upregulation of anti-inflammatory cytokines (IL-10 and TGF-β) and downregulation of proinflammatory cytokines (such as IL-1β, TNF-α) in macrophages [33]. Loss of GPR37 was found to prolong inflammatory pain and infection-induced pain in knockout mice [33, 75]. Artisunate, an anti-malaria drug, also serves as a GPR37 agonist that can promote macrophage phagocytosis and protect against sepsis and infection-induced pain [75]. Activation of the G-protein-coupled receptor kinase 2 (GRK2) in LysM (+) myeloid cells was also shown to induce the secretion of IL-10 and regulate inflammatory pain resolution. Loss of GRK2 in myeloid cells resulted in decreased macrophage IL-10 production and enhanced IL-1β-induced inflammatory hyperalgesia duration, which can be rescued via the adoption of wild-type bone marrow-derived monocytes [90]. Regular swim exercise has been shown to prevent persistent muscle hyperalgesia in rodents by activation of PPARγ receptors and modulation of macrophages phenotypes, leading to increased anti-inflammatory responses in the muscle tissue [91].
Recent findings of novel signaling mechanisms of macrophages in pain modulation
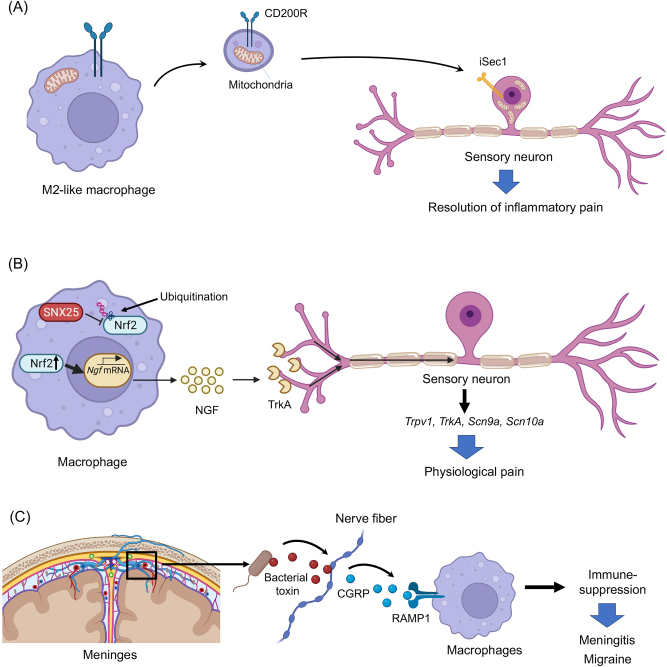
Recent studies also revealed novel signaling of macrophages in regulating pain resolution and homeostasis (Figure 5A–C). van der Vlist et al. found that M2-like macrophages infiltrate and accumulate in the DRG during the resolution phase of inflammatory pain in mice. Interestingly, these M2-like macrophages can transfer mitochondria to sensory neurons to alleviate inflammatory pain [92]. The CD200R (CD200R) receptor is located on macrophages, while the non-canonical CD200R-ligand iSec1 is located on sensory neurons. Both of these receptors are necessary for mitochondrial transfer and pain resolution (Figure 5A).
Figure 5:
New mechanisms of macrophage signaling in pain regulation. (A) Macrophage mitochondria transfer to neurons in DRG contributes to the resolution of inflammatory pain [92]. (B) Dermal macrophages set pain sensitivity in the physiological conditions by controlling the amount NGF in the skin [93]. (C) Meningeal macrophages protect infection and pain via CGRP signaling. This protective mechanism is hijacked by bacteria, leading to meningitis and headache [94].
Tanaka et al. showed that dermal macrophages set pain sensitivity by modulating the amount of tissue nerve growth factor (NGF) through sorting nexin (SNX)25 in dermal macrophages (SNX25) (Figure 5B) [93]. NGF is a critical regulator of nociceptor gene expression and drives inflammatory pain via regulation of the expression and function of TRPV1 [95–98] and sodium channels. Expression of SNX25 in dermal macrophages enhances expression of NGF through the inhibition of ubiquitin-mediated degradation of Nrf2, a transcription factor that activates transcription of NGF. Conditional deletion of SNX25 in monocytes and macrophages, but not in peripheral sensory neurons reduced pain responses in both normal and neuropathic conditions in conditional knockout mice [93].
Pinho-Ribeiro et al. revealed a meningeal neuroimmune axis by which bacteria activate trigeminal nociceptors to facilitate brain invasion and induce meningitis via CGRP-mediated inhibition of macrophage activation [94] (Figure 5C). It is well known that the meninges are densely innervated by nociceptive sensory neurons that can trigger trigeminal pain (headache and migraine) [99]. Bacterial infections by Streptococcus pneumoniae and Streptococcus agalactiae. S. cause life-threatening meningitis. Strikingly, these bacteria can directly activate nociceptors through their pore-forming toxin pneumolysin, leading to the release of CGRP. Furthermore, meningeal macrophages express CGRP receptor RAMP1 and activation of RAMP1 by CGRP can suppress macrophage’s inflammatory response (chemokine expression, neutrophil recruitment and antimicrobial defense) [94].
Guimaraes et al. demonstrated that nerve injury induced neuron-associated macrophage proliferation in DRG, which requires CX3CR1 signaling [100]. CX3CR1 in macrophages/monocytes was previously implied in the development of neuropathic pain [101]. Interestingly, the team found that nerve injury-induced increase in macrophage abundance is caused by the proliferation of resident CX3CR1+ macrophages but not the infiltration of peripheral monocytes from the circulation. Furthermore, this proliferation of resident macrophages contributes to the development of neuropathic pain in rodents by producing TNF and IL-1β [100].
Notably, most tissues and organs host their own resident macrophages, which have adapted to the local conditions to play tissue-specific roles in maintaining homeostasis [102]. These resident macrophages include Alveolar macrophages (lung), Kupffer cells (liver), perivascular macrophages (brain), and osteoclasts (bone), as well as dermal macrophages, peritoneal macrophages, and DRG macrophages. Osteoclasts are responsible for bone resorption. These multinucleated and giant cells are formed by fusion of osteoclast precursors following the activation of macrophage colony‐stimulating factor (M-CSF, also known as CSF-1) and RANKL (receptor activator of NF‐κB ligand). Osteoclasts play a critical role in cancer-induced bone pain via secreting pronociceptive mediators such as H+, proteases (cathepsin K and matrix metalloproteinases), and pro-inflammatory cytokines [45, 103]. Osteoclasts may also secrete netrin-1 to modulate axonal growth of sensory neurons. Subchondral bone osteoclasts were found to drive osteoarthritis pain via inducing sensory nerve innervations in bone tissue. Inhibition of osteoclast formation via RANKL knockout in osteocytes not only inhibited sensory nerve innervations into subchondral bone but also reduced neuronal hyperexcitability and pain hypersensitivity in mice with osteoarthritis [104]. The immune checkpoint pathway programed death protein 1 (PD-1) and its ligand PD-L1 has been implicated in the genesis of osteoclasts, bone destruction, and bone cancer pain. PD-L1 induced CCL2 production in osteoclasts, and CCR2 antagonist effectively suppressed bone cancer pain in mice [105]. Thus, immunotherapy through immune checkpoint inhibitors may alleviate cancer pain via suppressing osteoclast genesis and cancer-induced bone destruction and chemokine production [105]. Recently, tumor-associated macrophages (TAM) were shown to promote de novo neurogenesis and cancer pain [104]. Thus, tissue specific macrophages are both protective (e.g., dermal macrophages in health [93]) or detrimental (e.g., osteoclasts and TAM in cancer) in pain modulation.
Sex-dependent macrophage signaling in pain
The National Institutes of Health Revitalization Act of 1993 mandates the inclusion of female participants in clinical studies. However, preclinical studies, which predominantly use male animals, do not have a similar requirement. This bias arises from the complexities associated with menstrual hormone fluctuations and the assumption that male results can be generalized to females [106–108]. This disparity is particularly pronounced in neuroscience, where male single-sex studies outnumber female single-sex studies by almost five to one [109].
In chronic pain research, this disparity is a major issue, as women suffer inordinately from certain chronic pain conditions as compared to men, with the majority of pain clinic patients being women [110, 111]. These conditions include chronic orofacial/temporomandibular disorders, chronic fatigue syndrome, fibromyalgia, interstitial cystitis, neuropathic pain, and rheumatoid arthritis [106, 112, 113]. Moreover, women often experience greater levels of pain, as well as more frequency and more enduring pain [114]. Additionally, some studies suggest women may respond more poorly to analgesia than men, specifically opioid-induced analgesia [115]. Although the estrous cycle in female animals is sometimes cited as a potential confounding factor, it has been found that baseline thermal and mechanical pain sensitivity is not affected by the estrous cycle. Recent evidence has revealed sex dimorphism in pain processing by immune cells, particularly macrophages and microglia. Here, we summarize the sex dimorphism in macrophage mediated signaling.
During chronic pain, there are no obvious sex differences in the total macrophage population. When examining the chemotherapy-induced peripheral neuropathy (CIPN) model, it was found that the chemotherapy drug paclitaxel led to an elevation of F4/80+ macrophages in the DRG of both male and female mice [116]. Emerging research suggests that male and female macrophages might employ different signaling pathways to modulate acute and chronic pain. These distinct signaling pathways may contribute to the observed sex differences in pain responses and sensitivity [117].
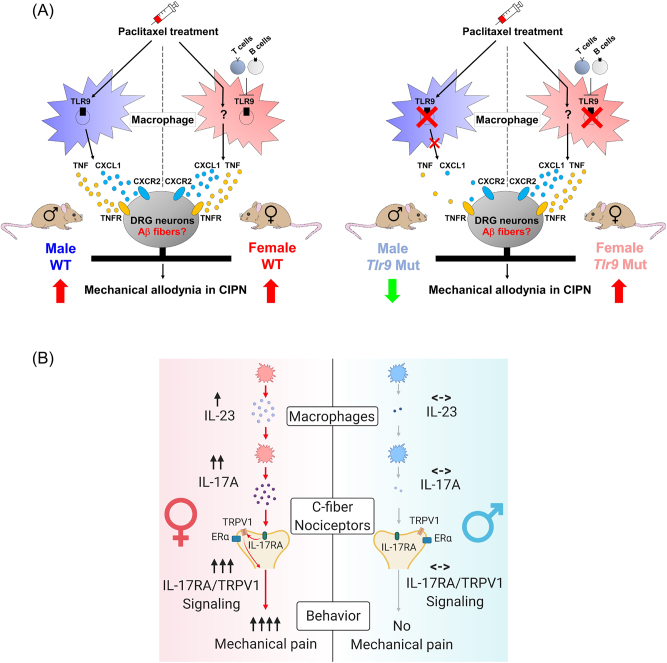
In males, studies have identified Toll-like receptor 9 (TLR9), colony stimulating factor-1 (CSF1), and high mobility group box 1 (HMGB1) as key components in the modulation of chronic pain in male mice [116, 118]. Male-specific TLR9 signaling was implicated in the development of chemotherapy-induced neuropathic pain [116]. TLR9 is typically localized in immune cells’ endolysosomes and detects bacterial DNA containing CpG motifs. Inhibiting TLR9 decreased mechanical allodynia in males but not females in a murine CIPN model. While adoptive transfer of paclitaxel-activated macrophages induced mechanical allodynia in both sexes, TLR9-deficinet macrophages induced less mechanical pain in males but not females. Mechanistically, macrophages produce cytokines and chemokines (e.g., TNF and CXCL1) to elicit pain. Treating primary macrophage cultures with paclitaxel resulted in increased TNF and CXCL1 production, but mutating Tlr9 inhibited this production in male cells but not female cells. Taken together, these results suggest that TLR9 signaling is central in male macrophages for pain processing (Figure 6A). In female mice with T cell deficiency, TLR9 inhibition was able to produce anti-nociceptive effects, suggesting that the regulation of the TLR9 pathways depends on T cells in females [116]. CSF1 is known to regulate the survival, proliferation, and differentiation of mononuclear phagocytes, such as macrophages. Yu et al. found that the neuronal depletion of Csf1 reduced the expansion of CX3CR1+ macrophages in DRGs of male mice following spared nerve injury (SNI), but this effect was not observed in female mice [118].
Figure 6:
Distinct macrophage signaling in pain in male and female mice. (A) Macrophage toll-like receptor 9 (TLR9) regulates mechanical pain in male mice. Left and right panels: schematic for macrophage TLR9 regulation of mechanical allodynia in chemotherapy-induced peripheral neuropathy (CIPN) in WT and Tlr9 mutant mice. Left, paclitaxel induces the infiltration and activation of macrophages in dorsal root ganglions (DRGs) in mice of both sexes. Paclitaxel also promotes macrophage release of TNF and CXCL1 in DRGs with subsequent binding to receptors TNFR1/R2 and CXCR2 on sensory neurons, thereby leading to hyperexcitability in nociceptive DRG neurons and driving mechanical allodynia in CIPN. Right, blocking TLR9 attenuates the development of paclitaxel (PTX)-elicited mechanical allodynia via downregulation of the PTX-induced macrophage release of TNF and CXCL1 only in male but not female mice. Additionally, female mice deficient in T and B cells may switch to utilizing TLR9 signaling in CIPN. Reproduced from Luo et al. [116] with permission (author’s own rights). (B) Macrophage signaling in female-dependent mechanical pain. Schematic illustration of female-specific modulation of mechanical pain by IL-23/IL-17A/transient receptor potential ion channel subtype V1 (TRPV1) axis. Note that both macrophage and nociceptor signaling contribute to the sex dimorphism. Reproduced from Luo et al. [119] with CCC permission.
HMGB1, a pro-inflammatory mediator that can be induced in DRG neurons by painful injury to activate TLR4 [120], was implicated in the development of chronic pain by promoting neuroinflammation [121]. Intra-articular injection of disulfide HMGB1 caused mechanical allodynia and increased proinflammatory cytokine expression in male but not female mice [122]. In primary macrophage cultures, disulfide HMGB1 triggers a higher release of TNF, IL-6, and CXCL1 in male cells compared to female cells. Co-injection of minocycline (an antibiotic that can inhibit monocytes and microglia) resulted in the reversal of pain hypersensitivity induced by disulfide HMGB1 in males only. Joint pain hypersensitivity induced by disulfide HMGB1 was reversed through selective Tlr4 depletion in LysM+ myeloid-derived cells in male mice only. Therefore, macrophage TLR4 may be required for HMGB1-mediated pain signaling in males [122].
In females, the interleukin-23/interleukin-17A (IL-23/IL-17A) axis has been shown to modulate mechanical pain in mice [119, 123] (Figure 6B). This female-specific role of the IL-23/IL-23R axis in pain processing involves a macrophage-dependent mechanism. IL-23-induced mechanical pain in females requires both macrophages and TRPV1+ C-fiber nociceptors. This sex-dependent mechanical pain is mediated by the pro-inflammatory cytokine, IL-17A, which is produced at higher levels in female macrophages (vs. male macrophages) and can directly activate nociceptors. By contrast, IL-23 does not directly activate nociceptors [119]. Female-specific mechanical pain by the IL-23/IL-17A axis is mediated by estrogen receptor alpha (ERα) in TRPV1+ nociceptors. Notably, IL-17A receptor (IL-17A) interacts with ERα and TRPV1 in DRG sensory neurons. In females, mechanical pain induced by IL-23, IL-17A, or capsaicin is inhibited by selective ERα depletion in TRPV1+ neurons in conditional knockout mice. These results point to the necessity of estrogen signaling in pain mediated by IL-23/IL-17A. ERα expression was observed to be higher in female samples of human DRG tissues compared to male samples [119]. Optogenetic stimulation of TRPV1+ nociceptors using blue light induced greater spontaneous pain in only female mice. Intraplantar IL-23 injection also potentiated blue light-induced pain in female mice but not in males [124].
Prolactin (PRL) was shown to produce female-specific pain via activation of PRL receptors on nociceptors [125–129]. A recent study has also implied female macrophages/monocytes in PRL-induced persistent pain [130]. Prolonged treatment of low dose PRL via intraplantar route caused non-resolving mechanical hypersensitivity only in female mice. Interestingly, this effect is independent of sensory neuronal PRL receptors and is associated with a lack of immune response in the hindpaw. Flow cytometry analysis revealed pro-inflammatory responses to macrophages/monocytes in hindpaw skins of females. It is suggested that a pro-inflammatory macrophage/monocyte-associated response in the hindpaws of mice is critical for the resolution of PRL-induced inflammatory pain of both sexes. In contrast, the lack of a peripheral inflammatory response contributed to PRL-induced persistent pain in females [130]. Together, these findings highlight the sex-specific mechanisms of pain modulation and the potential importance of considering these differences when developing pain management strategies.
Role of microglia in neuroinflammation and pain
Neuroinflammation is an inflammation of the nervous system and associated with a wide range of neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [5, 131–133]. Increasing literature suggests that neuroinflammation drives chronic pain (Figure 7A and B). Microglia, in particular, are a critical contributor to neuroinflammation during the development of chronic pain. As resident immune cells of the CNS, microglia migrate to the CNS during embryonic development. Once in the CNS, they differentiate and become a highly specialized cell type that can sense and respond to various stimuli [42]. Different cellular markers have been used to characterize microglia/myeloid cells, such as IBA1 and CX3CR1 for pan microglia (also for macrophages), P2RY12 and TMEM119 for resting microglia and CD68 for reactive microglia (also for macrophages) [134]. During neuroinflammation, activated microglia exhibited an amoeboid shape and increased expression of cell surface markers (e.g., CD11b). Activated microglia release various pro-inflammatory cytokines (TNF-α, IL-1β, IL-18, and IL-6), chemokines, and reactive oxygen species (ROS), which can cause neuronal damage, disrupt neuronal communication and induce pain. In turn, pro-inflammatory cytokines can further activate microglia in neuropathic pain [135]. In addition to their pro-inflammatory role, microglia also have anti-inflammatory and protective role in the CNS [42]. They can phagocytose and clear cellular debris, dead cells, and protein aggregates, which can prevent the spread of damage and promote tissue repair. They also produce anti-inflammatory cytokines, such as IL-10, which can dampen the inflammatory response and resolve pain. Especially, microglia express SPM receptors, such as ChemR23/ERV01 (resolvin E1 receptor), GPR32/DRV1 (resolvin D1 receptor), and GPR18 (resolvin D2 receptor) for the control of neuroinflammation and neuropathic pain [42, 136–138]. For example, RvE1 was found to reduce neuropathic pain via inhibiting p38-mediated TNF-α release in microglia [137]. Aspirin-triggered RvD1 was shown to induce autophagy and inhibit NLRP3 inflammasome in activated microglia [139].
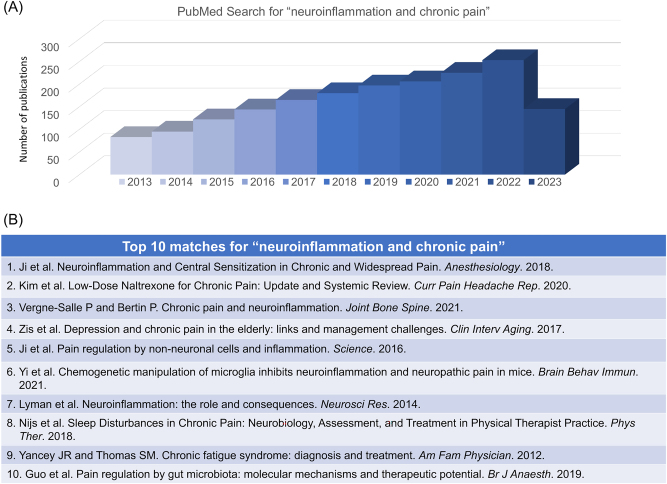
Figure 7:
PubMed search for “neuroinflammation and chronic pain”, conducted on July 15, 2023. (A) Distribution of the related papers in the past 10 years. This search shows a total of 2,154 publications. (B) Top 10 matched papers: 1) Ji et al. [4]; 2) Kim et al. [140]; 3) Vergne-Salle et al. [141]; 4) Zis et al. 2017; 5) Ji et al. [25]; 6) Yi et al. [142]; 7) Lyman et al. [143]; 8) Nijs et al. [144]; 9) Yancey JR and Thomas SM [145]; 10) Guo et al. [146].
Despite similar roles of macrophages and microglia in phagocytosis, microglia have limited capacity for this function as resident immune cells [147]. Understanding the role of microglia in neuroinflammation is essential for the development of effective treatments for multiple neuroinflammatory diseases, including pain.
Microglia in physiological states
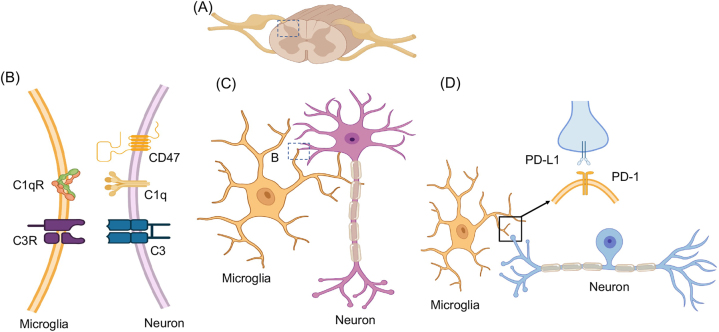
Growing evidence indicates that microglia display heterogeneity across the CNS and actively contribute to maintaining normal physiological states. By using their branched processes to monitor the cellular environment, microglia can swiftly alter their morphology in response to signaling molecules like ATP [148]. Microglia exhibit a range of morphologies depending on their activation state and the local environment. In their resting or surveilling state, microglia display a ramified morphology, characterized by a small cell body with long, branched processes extending in all directions. This morphology allows microglia to continuously survey their surroundings and detect any changes or damage [149, 150]. Upon activation, microglia can transform into an amoeboid shape, which is more suited for phagocytosis, synaptic pruning, and migration. During development or disease, microglia play a crucial role in refining neural circuits by pruning excess or unnecessary synapses, thereby optimizing neuronal connections, which involves the activation of the classic complement cascade through neuronal C1q and microglial C1 and C3 receptors [151, 152] (Figure 8A–C). Recently, immune checkpoint PD-L1/PD-1 pathway was implicated in pain, analgesia, anesthesia, cognition, and neurological disorders [153, 154]. In the spinal cord, primary afferent neurons (central terminals) express PD-L1, whereas microglia express PD-1. This unique ligand-receptor expression pattern may serve as a platform for neuron-microglia interaction in physiological and pathological pain states (Figure 8D).
Figure 8:
Microglia and neuron interactions in the spinal cord. (A) Schematic of spinal cord and dorsal root ganglia (DRG). (B and C) Microglia-neuron interactions through C1q-C1qR and C3 and C3R pairs. Note that CD47 is an inhibitory signal. (D) Interaction of microglia-death protein (PD)-1 receptor with axonal PD-L1 ligand from DRG neuron. Also see the role of the PD-L1/PD-1 axis in neurological diseases [153].
Direct membrane-membrane contacts between microglial processes and synaptic components have been confirmed through electron microscopy, in vivo 2-photon imaging, confocal laser scanning microscopy, and electron microscopy [155, 156]. A sophisticated study employed both confocal laser scanning microscopy and scanning electron microscopy that revealed microglia tend to interact more with presynaptic elements than dendritic spines [157]. Various molecular mechanisms have been identified in microglia-neuron communication at synapses, including the complement system, chemokine receptor pathway, and other interactions. Despite this, only a few markers have been found at these sites using targeted immunolabeling and advanced microscopy. Key molecules such as C1q, C3, CD47, and MHC class I have been observed in different contexts, playing roles in synapse elimination, protection, and regulation of synaptic plasticity (Figure 8B).
Complement component 1q (C1q) is necessary for synapse elimination, as driven by microglia. C1q can be found in developing synapses [158] and is involved in modulating spinal cord synaptic spine morphogenesis in inflammatory pain [159]. In some cases, complement component 3 (C3) was found to colocalize with Homer1, a synaptic marker and Complement C3-deficient mice showed improvement in age-related decline in hippocampal function [160]. Presynaptic vGluT2 and postsynaptic Homer1 were used to detect CD47 in presynaptic terminals. This transmembrane protein can protect synapses against unrestrained synaptic pruning by microglia [161]. Moreover, molecules of major histocompatibility complex class I (MHC class I) was involved in the modulation of synaptic plasticity and found to colocalize with the postsynaptic protein PSD-95 [162].
Microglia in the development of chronic pain
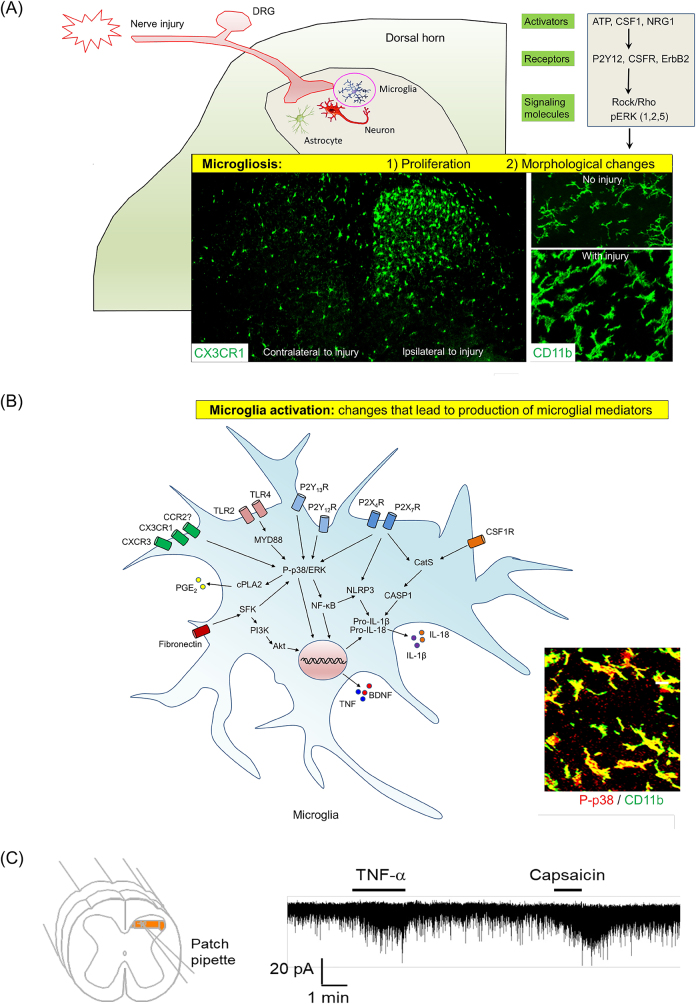
In the last two decades, major research progress has been made in demonstrating crucial roles of microglia in the pathogenesis of pain (Figure 9A and B). Spinal cord microglia are strongly activated after peripheral nerve injury, which causes the microglia to increase in size, shorten cellular processes, and change from a branching shape (ramified) to a blobby shape (ameboid). Nerve injury also results in an increased number of microglia (proliferation) in the first week [163–165]. These microglial changes in proliferation and morphologies are called microgliosis [165–167] (Figure 10A). Microgliosis may not be required or sufficient for the genesis of pain [41, 168]. However, microglia activation is sufficient to elicit and potentiate pain states [42] (Figure 10B). In 2003, three studies from different groups revealed an important role of spinal cord microglia in the development of neuropathic pain following nerve injury [169–171]. Several approaches, such as microglia ablation by toxin and inhibitor, as well as optogenetic and chemogenetic manipulations of microglia have shown that microglia are both sufficient and required for the induction of pain [142, 172–175].
Figure 9:
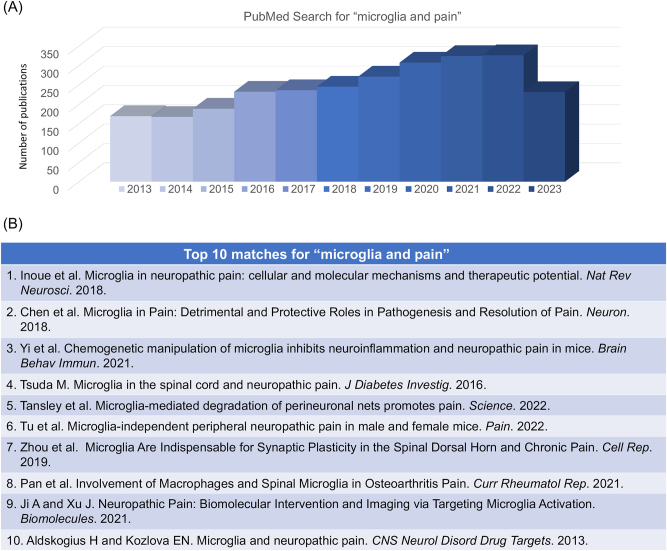
PubMed search for “microglia and pain” conducted on July 15, 2023. (A) Distribution of the related papers in the past 10 years (2013–2023). This search shows a total of 3,056 publications. (B) Top 10 matches. 1) Inoue et al. [176]; 2) Chen et al. [42]; 3) Yi et al. [142]; 4) Tsuda M. [177]; 5) Tansley et al. [178]; 6) Tu et al. [179]; 7) Zhou et al. [180]; 8) Pan et al. [181]; 9) Ji A and Xu J [182]; 10) Aldskogius H and Kozlova EN [183].
Figure 10:
Microglia in the development of neuropathic pain. (A) Nerve injury-induced microgliosis (proliferation and morphological changes). (B) Nerve injury-induced microglia activation, as indicated by upregulation and activation of ATP receptors, chemokine receptors, and TLRs (TLR4 and TLR2) [171, 184–188] and phosphorylation of MAP kinases p38 and ERK (1/2, 5) [169, 189–192]. As a result of microglia activation, pro-inflammatory mediators, such as TNF, IL-1β, IL-18, and PGE2, as well as growth factor (BDNF), are produced and secreted from microglia, inducing central sensitization in the spinal cord and enhancing pain states [151, 193–195]. A and B are reproduced from Chen et al. [42] with CCC permission. (C) Patch clamp recording in spinal cord slice reveals rapid increase in excitatory post-synaptic currents in spinal cord pain circuit following TNF treatment. The same neuron also responded to TRPV1 agonist capsaicin. Reproduced from Park et al. [196] with permission (author’s own right).
Activation of primary afferents such as C-fibers may be sufficient for triggering spinal microglial activation [197, 198]. However, large A-fiber activation also plays a significant role in sustaining microglial activation [199]. Guan et al. found that following peripheral nerve injury, colony stimulating factor 1 (CSF1) levels increase in damaged DRG neurons and CSF1 release from the injured primary afferent central terminals induces spinal microgliosis and behaviors relating to pain [200]. Interventions such as spinal injection of a CSF1 inhibitor or Cre-mediated deletion of Csf1 in DRG neurons can effectively mitigate microgliosis resulting from nerve injury as well as mechanical allodynia. Additionally, intrathecal injection of CSF1 in naive mice results in mechanical hypersensitivity and microgliosis, further highlighting the role of CSF1 in these processes [200]. Following peripheral nerve injury, sensory neurons also secrete chemokines such as CXCL1 (fractalkine) to activate microglia via CX3CR1 [201, 184]. CX3CR1 is exclusively expressed in the spinal cord microglia and its expression increases following nerve injury and arthritis [184, 202]. Microglia activation and mechanical allodynia development were markedly reduced in Cx3cr1 knockout mice after nerve injury [203]. Moreover, the protease cathepsin S is needed for the cleavage of CXCL1 from the DRG cell surface [204]. Additionally, peripheral nerve injury leads to the release of Neuregulin-1 from primary afferents, which activates ErbB receptors on spinal microglia. This activation contributes to the development of microgliosis and pain hypersensitivity through the phosphorylation of ERK1/2 and AKT signaling pathways in microglia [205]. Neuronal proteases are also crucial in microglia activation. A rapid and short-term increase in the expression of metalloproteinase-9 (MMP-9) in DRG neurons was found to induce microglial activation and trigger neuropathic pain [206]. Intrathecal MMP-9 administration leads to IL-1β activation via cleavage, causing symptoms of neuropathic pain as well as microgliosis. While this increase is involved in the onset of neuropathic pain, it is not required for maintaining neuropathic pain [206]. Especially, caspase 6 is expressed in axons and involved in axonal degeneration [207]. Nerve injury was shown to induce caspase 6 upregulation in injured sensory neurons [208]. Furthermore, caspase 6 release from damaged central axons is sufficient to activate microglia through p38 phosphorylation and trigger TNF-α release, causing TNF-induced pain [209] (Figure 10B).
Microglia activation is accompanied by swift activation of intracellular signaling pathways, such as the phosphorylation of MAP kinases like p38 and ERK [189, 210]. The pharmacological inhibition of p38 and ERK can reduce microgliosis and pain hypersensitivity following peripheral nerve injury [211, 212]. Microglial intracellular signaling cascade activation accommodates upregulations of microglial cell-surface receptors in the spinal cord dorsal horn, including toll-like receptors (TLRs), purinergic receptors, and chemokine receptors [176]. The TLR family plays a critical role in innate immune response and pathogenesis of pain [213]. Extensive research on TLR4 in nerve injury-induced neuropathic pain has demonstrated its critical role in microglial activation and cytokine expression [185, 214]. Knocking out of Tlr2 was also shown to decrease neuropathic pain and spinal microglia activation [186]. One powerful microglia activator is ATP, which can be produced from different cell types after nerve injury, including microglia, astrocytes, and neurons in the spinal cord [215–217]. In spinal cord microglia, the ATP purinoceptors P2X4R and P2X7R are both upregulated following nerve injury. Blocking the signaling of these purinoceptors in the spine leads to reductions in BDNF release and mechanical allodynia after nerve injury [171, 218]. In murine neuropathic pain models, P2X4R and P2X7R knockout or knockdown led to significant attenuation of neuropathic pain [171, 219–222]. In addition, spinal microglia also express P2Y receptors, including P2Y6R, P2Y12R, P2Y13R, and P2Y14R, and these P2Y receptors also contribute to neuropathic pain development [190, 223, 224].
TNF-α (or TNF) is one of the most prominent microglial mediators. Peripheral mechanisms of TNF in the pathogenesis of pain have been well studied, and TNF is sufficient to induce hyperexcitability of primary sensory neurons [62, 225, 226]. TNF also drives central sensitization in spinal cord pain circuit [193]. Patch clamp recordings in spinal cord slices revealed that TNF perfusion induced rapid increase, within minutes, in excitatory postsynaptic currents (EPSC) (Figure 10C) [193, 196, 227]. TNF further induces long-term potentiation (LTP) in spinal cord neurons, a crucial cellular mechanism for the development of persistent pain, via TNFR1 and TNFR2 [196, 228, 229]. Notably, SPMs, such as resolvin E1 and protectin D1/neuroprotectin D1, can potently inhibit TNF-induced synaptic plasticity in the spinal cord pain circuit [82, 230]. Additionally, miRNAs have been implicated in microglia signaling in neuropathic pain [231, 232].
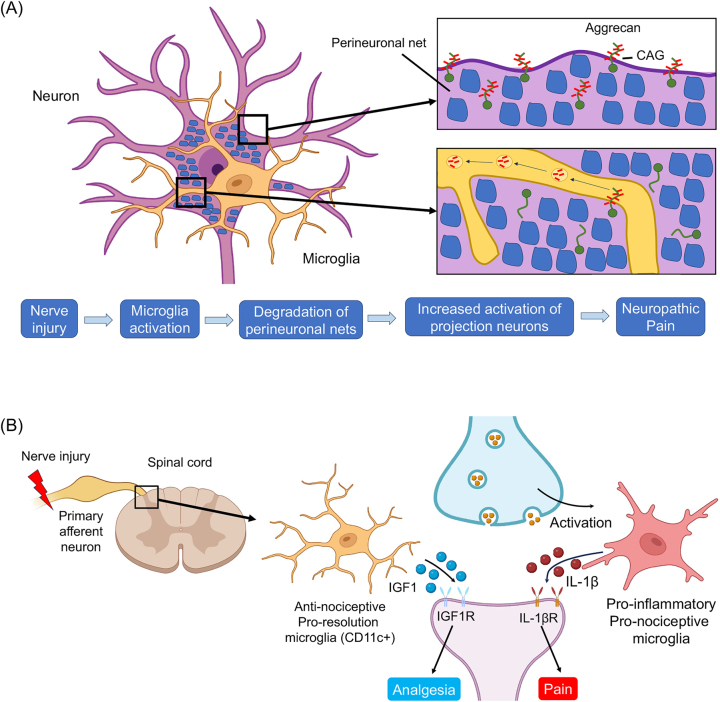
In 2022, researchers demonstrated additional microglial signaling in neuropathic pain. First, microglia-mediated degradation of perineuronal nets promotes neuropathic pain in mice [178]. Following peripheral nerve injury, activated microglia degraded extracellular matrix structures, perineuronal nets (PNNs), in lamina I projection neurons of the spinal cord dorsal horn. Degradation of PNNs by microglia resulted in enhanced activity of projection neurons and enhanced neuropathic pain [178] (Figure 11A). Second, the Alzheimer’s disease gene Apoe (encoding Apolipoprotein E) is also the top upregulated gene in spinal cord microglial cells at chronic time points following peripheral nerve injury in mice. Genetic analysis revealed polymorphisms in the APOE gene in humans are associated with chronic pain [233]. Third, microglia drive chronic pain in the SNI model (>14 months) via telomere- and p53-mediated spinal cord cellular senescence, and chronic pain is associated with reduced life span in mice [234].
Figure 11:
Novel microglial signaling in the development and resolution of neuropathic pain. (A) Following nerve injury microglia activation induces neuropathic pain via degradation of peri-neuronal nets, leading to the activation of projection neurons in the superficial spinal cord. See more details in Tansley et al. [178]. (B) Microglia regulate the resolution of neuropathic pain. Nerve injury induces CD11c population of microglia in the late phase (three weeks), which contributes to the resolution of neuropathic pain via IGF-1. Note microglia can induce both pain and analgesia through different mediators, which act on nociceptive neurons. See more details in Kohno et al. [235].
Microglia in the resolution of pain
While microglia play a vital role in neuroinflammation and the development of neurological and neuropsychiatric diseases, they help maintain brain and spinal cord homeostasis and resolve neuroinflammation. Recently, many genes in microglia were found to be correlated with their protective effects in neuronal diseases. Notably, when compared to their wild-type counterparts, mice lacking the Cx3cr1 gene showed more severe cognitive dysfunction and greater levels of neuronal death with traumatic brain injury in the chronic phase [42]. The contrasting behavioral outcomes in the acute vs. chronic phases of brain injury in Cx3cr1-deficient mice could be related to different microglial phenotypes present during these stages.
Kohno et al. discovered a group of CD11c+ microglia that appear during pain maintenance and aid in the reduction of neuropathic pain following peripheral nerve injury [235, 236] (Figure 11B). When peripheral nerve injury occurs, microglial activation in the dorsal horn initiates pain-promoting communication with neurons by releasing cytokines such as TNF-a and IL-1β, which enhance neuronal activity. During pain maintenance, a group of CD11c+ microglia, expressing the receptor AXL emerges, and engulf myelin debris from deteriorating processes impacting injured neurons. These phagocytic CD11c+ microglia secrete insulin-like growth factor 1 (IGF-1), which counteracts pain through the activation of neuronal IGF-1 receptor (IGF1R). This finding challenges the existing understanding of microglia’s role in chronic pain and highlights the need to reassess the potential of microglia as a therapeutic target. By understanding the role of IGF1, APOE, and the context required for the emergence of antinociceptive microglia, new therapeutic strategies can be designed to promote analgesia and potentially alleviate chronic pain. However, the pronociceptive role of IGF1 has also been shown in DRG neurons [237]. It remains to be tested whether IGF1 plays a distinct role in the PNS and CNS.
Activation of the cannabinoid receptor type 2 (CB2) promotes an anti-inflammatory microglial phenotype by increasing the expression of MAP kinase phosphatase and suppressing MAP kinase phosphorylation, which is crucial for alleviating neuropathic pain [41, 238]. Interestingly, CB2 expression could be upregulated in chronic pain states [239–241]. Given extensive medical and recreational use of cannabis and related products, understanding the mechanisms of endocannabinoids and exogenous cannabinoids in glial and immune cells in acute and chronic pain has great social and clinical impact. The anti-inflammatory cytokine IL-10, likely a microglial product, has been found to inhibit microgliosis and neuropathic pain following nerve injury in early life [242]. Recently, it was found that high-intensity swimming reduced ischemic pain and neuroinflammation in mice by resolvin E1 mediated activation of ChemR23 and anti-inflammatory polarization in spinal microglia [243].
Sex-dependent and sex-independent regulation of microglia in pain
Studies from different lab have shown that nerve injury induces similar morphological changes in spinal cord microglia (microgliosis) in both sexes [42, 244–246]. Nonetheless, many studies have highlighted a male-dominant role of microglia in regulating pathological pain, especially mechanical allodynia, across various chronic pain conditions. These findings have been gathered through transgenic and pharmacological approaches, such as selectively activating or depleting microglia, employing a general microglial inhibitor to hinder their function, and specifically inhibiting microglial signaling pathways [42].
Using microglia manipulations done through a chemogenetic approach called DREADD (Designer Receptor Exclusively Activated by a Designer Drug) and intrathecal administration of C-N-oxide (CNO), it was found that selective activation of spinal microglia resulted in mechanical allodynia in male rats and mice, while female counterparts failed to exhibit the same response [174, 247]. Furthermore, microglia of male rats were transfected with inhibitory Gi DREADDs and intrathecal administration of CNO in these male rats led to a reduction in the allodynia [174]. This finding indicates that the activation of inhibitory Gi DREADDs in spinal microglia can help alleviate CCI-induced allodynia in male subjects not female. However, ablation of spinal microglia was also shown to reduce neuropathic pain in both sexes [248].
The p38 protein is an important member of the MAPK family and expressed by microglia in the spinal cord. Injecting the p38 inhibitor skepinone into the spinal canal only alleviates CCI-induced mechanical allodynia in male mice, whereas injecting it systemically or around nerves provides pain relief in both sexes, implying different mechanisms for peripheral and central p38 pathways [245]. CCI-induced mechanical allodynia is diminished in male mice only following intrathecal injection of p38α antisense oligonucleotides (ASO), leading to approximately a 50 % reduction in spinal levels of p38α mRNA [249]. In a mouse model of hyperalgesic priming, the p38 inhibitor skepinone blocks sustained mechanical allodynia caused by PGE2 exclusively in male animals [250].
The microglia inhibitor, minocycline, only produces antinociceptive and anti-allodynic effects in male mice in several pain models, such as the spared nerve injury model (SNI) [172], the formalin model [251], the chronic constriction injury model (CCI) [245, 246, 251], and the collagen induced arthritis model (CIA) (206). Moreover, intrathecal administration of minocycline enhances morphine analgesia in male rats, but not in females [252]. Notably, minocycline is not a selective inhibitor of microglia. It produces a number anti-inflammatory actions, including inhibition of MMPs (e.g., MMP-9), inhibition of neutrophil migration and chemotaxis, and inhibition of proinflammatory cytokines (e.g., IL-1α/β, IL-6, IL-8, TNF-α). However, at high concentrations, minocycline may also affect astrocytes, oligodendrocytes, and neurons [209, 253]. It was also found that microglia depletion by using saporin toxin conjugated with macrophage antigen complex-1 (Mac-1) only resulted in reversal of mechanical allodynia in male mice, not in female mice [172]. Neuronal caspase-6 plays a crucial role in regulating microglial function in chronic pain. Caspase-6 inhibitor or caspase6 knockout led to male-dominant analgesia in inflammatory and neuropathic pain models [209, 254]. Sorge et al. identified TLR4, which is critical for producing inflammatory cytokines from microglia [255], contributed to mechanical allodynia solely in males [256]. Microglial P2X4-BDNF pathway also regulates neuropathic pain in male mice [172, 246]. In contrast, nerve injury-induced spinal microgliosis exhibited no sex differences [245, 246]. Furthermore, microglia were found to drive chronic pain in the SNI model of male mice via a cellular senescence pathway, and chronic pain is associated with reduced life span in males [234].
Conclusion remarks and future directions
Neuroimmune interactions are emerging as a central topic of pain research. Diverse types of non-neuronal cells have been implicated in physiological and pathological pain. As the most investigated immune cell type in pain modulation, macrophages play important roles not only in the induction of pain but also in the resolution of pain. As resident macrophages in the CNS and the most investigated glial cell type in pain modulation, microglia are activated by nerve injury and contribute critically to the development of neuropathic pain. Macrophages and microglia are critical regulators of inflammation and neuroinflammation. Especially, neuroinflammation in the PNS and CNS is highly correlated with chronic pain [4]. It is difficult to clearly separate the roles of macrophages and microglia in pain, as they express common cellular markets such as CX3CR1. Functionally, macrophages and microglia can co-operate to regulate chronic pain. Accumulating evidence indicates that macrophages and microglia regulate diverse types of pain conditions, ranging from physiological pain under the homeostasis, to pathological pain induction and maintenance, and resolution of acute and persistent pain. Recent advances in neuroimmune and neuroglial interactions not only provide new mechanistic insights into acute and chronic pain, but also open avenues for the treatments of chronic pain. Accumulating evidence has also revealed microglia activation in the pain-modulating brain regions (e.g., the thalamus) in patients with chronic pain [257]. Future studies are needed to investigate the role of macrophages and microglia in clinical pain.
In addition to macrophages and microglia, other non-neuronal cell types have been implicated in pain, including but not limited to astrocytes, satellite glial cells, Schwann cells, neutrophils, and T cells [25, 258–262]. Immunotherapy has achieved great success in treating cancers, saving the lives of millions of patients [263–265]. Immunotherapy has also been proposed for the treatment of cognitive declines in animal models of Alzheimer’s disease and brain injury [154, 266]. We highlighted immunotherapy for the management of chronic pain [267], such as bone cancer pain with immune checkpoint inhibitors and STING agonists [105, 268, 269], or arthritic pain using monoclonal antibodies against inflammatory cytokines and their receptors [270], or neuropathic and inflammatory pain using cell therapies (e.g., mesenchymal stromal/stem cells) [86, 271]. The emerging regenerative pain medicine includes cell products and blood products, such as platelet-rich plasma (PRP) and autologous conditioned serums (ACS) [272, 273]. Strikingly, intrathecal adoptive transfer of human conditioned serum to mice with neuropathic pain elicited sustained pain relief for many weeks. It was found that conditioned serum contains higher levels of exosomes than non-conditioned serum and the secreted exosome are essential for the long-term analgesia by this regenerative medicine and immunotherapy, as this treatment can modulate neuroinflammation in the spinal cord [274]. Exosomes released from macrophages or stem cells were protective against inflammatory pain in mice [8, 275]. It is of great interest to compare the therapeutic effects of small vesicles/exosomes of different origins (macrophages, microglia, stem/stromal cells, ACS) in chronic inflammation and chronic pain conditions. Macrophage polarization can be modulated by SPMs [80, 37] and iron-based nanoparticles [76]. Control of macrophage or microglia polarization in vitro or in vivo through “immune resolution” or “immune rejuvenation” may serve as novel therapeutic strategy for pain management.
Footnotes
Research ethics: This is a review article. The local Institutional Review Board deemed the study exempt from review.
Informed consent: Informed consent was obtained from all individuals included in this study. The authors received permission (CCC license) for the reuse of the published figures. Other figures are prepared by the authors and have not been published.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Ru-Rong Ji is an editorial board member of Medical Review and does not participate in the peer review and decision-making of articles.
Research funding: This study is supported by Duke University Research Funds.
Data availability: This is a review article, and no original data are presented. All the data used in this review article are available upon the request to the corresponding author (ru-rong.ji@duke.edu).
References
- 1.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–6. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Ji JYM, Ji RR. Neuroimmune interactions in pain. Switzerland: Springer Cham; 2023. Inflammation and pain; pp. 17–41. [Google Scholar]
- 3.Harth M, Nielson WR. Pain and affective distress in arthritis: relationship to immunity and inflammation. Expert Rev Clin Immunol. 2019;15:541–52. doi: 10.1080/1744666x.2019.1573675. [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018 doi: 10.1097/aln.0000000000002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–48. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer C, Leinders M, Uceyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159:595–602. doi: 10.1097/j.pain.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 7.Bjorklund G, Aaseth J, Dosa MD, Pivina L, Dadar M, Pen JJ, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153–65. doi: 10.1016/j.nut.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 8.McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R, et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain. 2014;155:1527–39. doi: 10.1016/j.pain.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482–503. doi: 10.1097/aln.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Hao C, Zhang Z, Jiang H, Zhang W, Huang J, et al. Shenjinhuoxue mixture attenuates inflammation, pain, and cartilage degeneration by inhibiting TLR-4 and NF-kappaB activation in rats with osteoarthritis: a synergistic combination of multitarget active phytochemicals. Oxid Med Cell Longev. 2021;2021:4190098. doi: 10.1155/2021/4190098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arango-Davila CA, Rincon-Hoyos HG. Depressive disorder, anxiety disorder and chronic pain: multiple manifestations of a common clinical and pathophysiological core. Rev Colomb Psiquiatr (Engl Ed) 2018;47:46–55. doi: 10.1016/j.rcpeng.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Santoni M, Miccini F, Battelli N. Gut microbiota, immunity and pain. Immunol Lett. 2021;229:44–7. doi: 10.1016/j.imlet.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–82. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 15.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–64. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–57. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–53. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 19.Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta-endorphin mediates addiction to UV light. Cell. 2014;157:1527–34. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci USA. 2013;110:E3225–34. doi: 10.1073/pnas.1312933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Wang ZL, Yeo M, Zhang QJ, Lopez-Romero AE, Ding HP, et al. Epithelia-sensory neuron cross talk underlies cholestatic itch induced by lysophosphatidylcholine. Gastroenterology. 2021;161:301–17. doi: 10.1053/j.gastro.2021.03.049. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang MD, Usoskin D, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365:695–9. doi: 10.1126/science.aax6452. [DOI] [PubMed] [Google Scholar]
- 23.Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–37. doi: 10.1523/jneurosci.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, et al. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6:1186–93. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–7. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Q, Dong X, Green DP, Dong X. Peripheral mechanisms of chronic pain. Med Rev (Berl) 2022;2:251–70. doi: 10.1515/mr-2022-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 2019;101:412–20.e3. doi: 10.1016/j.neuron.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao C, Chen O, Sheng H, Zhang J, Luo Y, Hayes BW, et al. A mast cell-thermoregulatory neuron circuit axis regulates hypothermia in anaphylaxis. Sci Immunol. 2023;8:eadc9417. doi: 10.1126/sciimmunol.adc9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanc P, Gonzalez RJ, Mazo IB, Wang Y, Lambert T, Ortiz G, et al. Multimodal control of dendritic cell functions by nociceptors. Science. 2023;379:eabm5658. doi: 10.1126/science.abm5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talbot S, Foster SL, Woolf CJ. Neuroimmunity: physiology and pathology. Annu Rev Immunol. 2016;34:421–47. doi: 10.1146/annurev-immunol-041015-055340. [DOI] [PubMed] [Google Scholar]
- 31.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 32.McMahon SB, La Russa F, Bennett DL. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat Rev Neurosci. 2015;16:389–402. doi: 10.1038/nrn3946. [DOI] [PubMed] [Google Scholar]
- 33.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest. 2018;128:3568–82. doi: 10.1172/jci99888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19:433–47. doi: 10.1038/s41577-019-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fattori V, Staurengo-Ferrari L, Zaninelli TH, Casagrande R, Oliveira RD, Louzada-Junior P, et al. IL-33 enhances macrophage release of IL-1beta and promotes pain and inflammation in gouty arthritis. Inflamm Res. 2020;69:1271–82. doi: 10.1007/s00011-020-01399-x. [DOI] [PubMed] [Google Scholar]
- 36.Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol. 2020;62:17–25. doi: 10.1016/j.conb.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Bang S, Chandra S, Ji RR. Inflammation and infection in pain and the role of GPR37. Int J Mol Sci. 2022;23:14426. doi: 10.3390/ijms232214426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domoto R, Sekiguchi F, Tsubota M, Kawabata A. Macrophage as a peripheral pain regulator. Cells. 2021;10:1881. doi: 10.3390/cells10081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Xie H, Yao S, Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. 2017;14:53. doi: 10.1186/s12974-017-0828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubisic V, McClain JL, Fried DE, Grants I, Rajasekhar P, Csizmadia E, et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 2020;32:108100. doi: 10.1016/j.celrep.2020.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(1 Suppl):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100:1292–311. doi: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 44.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–6. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andriessen AS, Donnelly CR, Ji RR. Reciprocal interactions between osteoclasts and nociceptive sensory neurons in bone cancer pain. Pain Rep. 2021;6:e867. doi: 10.1097/pr9.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain. 2013;154(1 Suppl):S54–62. doi: 10.1016/j.pain.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 47.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji RR, Lee SY. Molecular sensors of temperature, pressure, and pain with special focus on TRPV1, TRPM8, and PIEZO2 ion channels. Neurosci Bull. 2021;37:1745–9. doi: 10.1007/s12264-021-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnelly CR, Chen O, Ji RR. How do sensory neurons sense danger signals? Trends Neurosci. 2020;43:822–38. doi: 10.1016/j.tins.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4:834–44. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn W, Chi G, Kim S, Son Y, Zhang M. Substance P reduces infarct size and mortality after ischemic stroke, possibly through the M2 polarization of microglia/macrophages and neuroprotection in the ischemic rat brain. Cell Mol Neurobiol. 2023;43:2035–52. doi: 10.1007/s10571-022-01284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma W, Chabot JG, Vercauteren F, Quirion R. Injured nerve-derived COX2/PGE2 contributes to the maintenance of neuropathic pain in aged rats. Neurobiol Aging. 2008;31:1227–37. doi: 10.1016/j.neurobiolaging.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Lin CR, Amaya F, Barrett L, Wang H, Takada J, Samad TA, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Therapeut. 2006;319:1096–103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 55.Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, et al. The complement system component C5a produces thermal hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. J Neurosci. 2016;36:5055–70. doi: 10.1523/jneurosci.3249-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warwick CA, Shutov LP, Shepherd AJ, Mohapatra DP, Usachev YM. Mechanisms underlying mechanical sensitization induced by complement C5a: the roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain. 2019;160:702–11. doi: 10.1097/j.pain.0000000000001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen LH, Yeh YM, Chen YF, Hsu YH, Wang HH, Lin PC, et al. Targeting interleukin-20 alleviates paclitaxel-induced peripheral neuropathy. Pain. 2020;161:1237–54. doi: 10.1097/j.pain.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 58.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102:14092–7. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richter F, Natura G, Ebbinghaus M, von Banchet GS, Hensellek S, Konig C, et al. Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis Rheum. 2012;64:4125–34. doi: 10.1002/art.37695. [DOI] [PubMed] [Google Scholar]
- 60.Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–6. doi: 10.1523/jneurosci.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–55. doi: 10.1523/jneurosci.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–55. doi: 10.1523/jneurosci.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/jneurosci.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiu IM, Heesters BA, Ghasemlou N, von Hehn CA, Zhao F, Tran J, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–7. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Boyette-Davis JA, Kosturakis AK, Li Y, Yoon SY, Walters ET, et al. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J Pain. 2013;14:1031–44. doi: 10.1016/j.jpain.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu XJ, Liu T, Chen G, Wang B, Yu XL, Yin C, et al. TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep. 2016;6:28188. doi: 10.1038/srep28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, et al. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 2014;24:1374–7. doi: 10.1038/cr.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie RG, Gao YJ, Park CK, Lu N, Luo C, Wang WT, et al. Spinal CCL2 promotes central sensitization, long-term potentiation, and inflammatory pain via CCR2: further insights into molecular, synaptic, and cellular mechanisms. Neurosci Bull. 2018;34:13–21. doi: 10.1007/s12264-017-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He L, Han G, Wu S, Du S, Zhang Y, Liu W, et al. Toll-like receptor 7 contributes to neuropathic pain by activating NF-kappaB in primary sensory neurons. Brain Behav Immun. 2020;87:840–51. doi: 10.1016/j.bbi.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qureshi RA, Tian Y, McDonald MK, Capasso KE, Douglas SR, Gao R, et al. Circulating microRNA signatures in rodent models of pain. Mol Neurobiol. 2016;53:3416–27. doi: 10.1007/s12035-015-9281-4. [DOI] [PubMed] [Google Scholar]
- 72.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, et al. Extracellular MicroRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simeoli R, Montague K, Jones HR, Castaldi L, Chambers D, Kelleher JH, et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat Commun. 2017;8:1778. doi: 10.1038/s41467-017-01841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeboudj L, Sideris-Lampretsas G, Silva R, Al-Mudaris S, Picco F, Fox S, et al. Silencing miR-21-5p in sensory neurons reverses neuropathic allodynia via activation of TGF-beta-related pathway in macrophages. J Clin Invest. 2023;133:e164472. doi: 10.1172/jci164472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bang S, Donnelly CR, Luo X, Toro-Moreno M, Tao X, Wang Z, et al. Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice. Nat Commun. 2021;12:1704. doi: 10.1038/s41467-021-21940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He D. Modulation of macrophage polarization by iron-based nanoparticles. Med Rev (Berl) 2023;3:105–22. doi: 10.1515/mr-2023-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol. 2015;51:19–31. doi: 10.1007/s12035-014-8790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119:414–7. doi: 10.1161/circresaha.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–65. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64:443–62. doi: 10.1042/ebc20200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–7.1. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest. 2019;129:5294–311. doi: 10.1172/jci129448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji RR. Specialized pro-resolving mediators as resolution pharmacology for the control of pain and itch. Annu Rev Pharmacol Toxicol. 2023;63:273–93. doi: 10.1146/annurev-pharmtox-051921-084047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei J, Su W, Zhao Y, Wei Z, Hua Y, Xue P, et al. Maresin 1 promotes nerve regeneration and alleviates neuropathic pain after nerve injury. J Neuroinflammation. 2022;19:32. doi: 10.1186/s12974-022-02405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin Invest. 2015;125:3226–40. doi: 10.1172/jci80883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, et al. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci. 2016;36:11074–83. doi: 10.1523/jneurosci.3708-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, et al. Monocytes/macrophages control resolution of transient inflammatory pain. J Pain. 2014;15:496–506. doi: 10.1016/j.jpain.2014.01.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baliu-Pique M, Jusek G, Holzmann B. Neuroimmunological communication via CGRP promotes the development of a regulatory phenotype in TLR4-stimulated macrophages. Eur J Immunol. 2014;44:3708–16. doi: 10.1002/eji.201444553. [DOI] [PubMed] [Google Scholar]
- 90.Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, Kelley KW, et al. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain. 2010;150:550–60. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Azambuja G, Jorge CO, Gomes BB, Lourenco HR, Simabuco FM, Oliveira-Fusaro MCG. Regular swimming exercise prevented the acute and persistent mechanical muscle hyperalgesia by modulation of macrophages phenotypes and inflammatory cytokines via PPARgamma receptors. Brain Behav Immun. 2021;95:462–76. doi: 10.1016/j.bbi.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 92.van der Vlist M, Raoof R, Willemen H, Prado J, Versteeg S, Martin Gil C, et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron. 2022;110:613–26.e9. doi: 10.1016/j.neuron.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka T, Okuda H, Isonishi A, Terada Y, Kitabatake M, Shinjo T, et al. Dermal macrophages set pain sensitivity by modulating the amount of tissue NGF through an SNX25-Nrf2 pathway. Nat Immunol. 2023;24:439–51. doi: 10.1038/s41590-022-01418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pinho-Ribeiro FA, Deng L, Neel DV, Erdogan O, Basu H, Yang D, et al. Bacteria hijack a meningeal neuroimmune axis to facilitate brain invasion. Nature. 2023;615:472–81. doi: 10.1038/s41586-023-05753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–62. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 96.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 97.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–9. doi: 10.1523/jneurosci.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, et al. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–10. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- 99.Levy D, Moskowitz MA. Meningeal mechanisms and the migraine connection. Annu Rev Neurosci. 2023;46:39–58. doi: 10.1146/annurev-neuro-080422-105509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guimaraes RM, Anibal-Silva CE, Davoli-Ferreira M, Gomes FIF, Mendes A, Cavallini MCM, et al. Neuron-associated macrophage proliferation in the sensory ganglia is associated with peripheral nerve injury-induced neuropathic pain involving CX3CR1 signaling. Elife. 2023;12:e78515. doi: 10.7554/elife.78515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Old EA, Nadkarni S, Grist J, Gentry C, Bevan S, Kim KW, et al. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest. 2014;124:2023–36. doi: 10.1172/jci71389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2:201–9. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 104.Zhu S, Zhu J, Zhen G, Hu Y, An S, Li Y, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129:1076–93. doi: 10.1172/jci121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest. 2020;130:3603–20. doi: 10.1172/jci133334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 107.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21:353–65. doi: 10.1038/s41583-020-0310-6. [DOI] [PubMed] [Google Scholar]
- 108.Navratilova E, Fillingim RB, Porreca F. Sexual dimorphism in functional pain syndromes. Sci Transl Med. 2021;13:eabj7180. doi: 10.1126/scitranslmed.abj7180. [DOI] [PubMed] [Google Scholar]
- 109.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults – United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:1001–6. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shupler MS, Kramer JK, Cragg JJ, Jutzeler CR, Whitehurst DGT. Pan-Canadian estimates of chronic pain prevalence from 2000 to 2014: a repeated cross-sectional survey analysis. J Pain. 2019;20:557–65. doi: 10.1016/j.jpain.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 112.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maixner W, Humphrey C. Gender differences in pain and cardiovascular responses to forearm ischemia. Clin J Pain. 1993;9:16–25. doi: 10.1097/00002508-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 114.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–67. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 115.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–25. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 116.Luo X, Huh Y, Bang S, He Q, Zhang L, Matsuda M, et al. Macrophage toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J Neurosci. 2019;39:6848–64. doi: 10.1523/jneurosci.3257-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo X. Sex differences in pain with emphasis on neuroimmune interactions. Neuroimmune interactions in pain. Switzerland: Springer Cham; 2023. pp. 153–70. [Google Scholar]
- 118.Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11:264. doi: 10.1038/s41467-019-13839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021;109:2691–706.e5. doi: 10.1016/j.neuron.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shibasaki M, Sasaki M, Miura M, Mizukoshi K, Ueno H, Hashimoto S, et al. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain. 2010;149:514–21. doi: 10.1016/j.pain.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 121.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, et al. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–44. doi: 10.1016/s0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 122.Agalave NM, Rudjito R, Farinotti AB, Khoonsari PE, Sandor K, Nomura Y, et al. Sex-dependent role of microglia in disulfide high mobility group box 1 protein-mediated mechanical hypersensitivity. Pain. 2021;162:446–58. doi: 10.1097/j.pain.0000000000002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tan Z, Lin ZJ, Wu LJ, Zhou LJ. The macrophage IL-23/IL-17A pathway: a new neuro-immune mechanism in female mechanical pain. Neurosci Bull. 2022;38:453–5. doi: 10.1007/s12264-021-00797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ji J, He Q, Luo X, Bang S, Matsuoka Y, McGinnis A, et al. IL-23 enhances C-fiber-mediated and blue light-induced spontaneous pain in female mice. Front Immunol. 2021;12:787565. doi: 10.3389/fimmu.2021.787565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patil MJ, Ruparel SB, Henry MA, Akopian AN. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab. 2013;305:E1154–64. doi: 10.1152/ajpendo.00187.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patil MJ, Green DP, Henry MA, Akopian AN. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience. 2013;253:132–41. doi: 10.1016/j.neuroscience.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Patil M, Belugin S, Mecklenburg J, Wangzhou A, Paige C, Barba-Escobedo PA, et al. Prolactin regulates pain responses via a female-selective nociceptor-specific mechanism. iScience. 2019;20:449–65. doi: 10.1016/j.isci.2019.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, et al. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–36. doi: 10.1523/jneurosci.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y, Moutal A, Navratilova E, Kopruszinski C, Yue X, Ikegami M, et al. The prolactin receptor long isoform regulates nociceptor sensitization and opioid-induced hyperalgesia selectively in females. Sci Transl Med. 2020;12:eaay7550. doi: 10.1126/scitranslmed.aay7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mecklenburg J, Wangzhou A, Hovhannisyan AH, Barba-Escobedo P, Shein SA, Zou Y, et al. Sex-dependent pain trajectories induced by prolactin require an inflammatory response for pain resolution. Brain Behav Immun. 2022;101:246–63. doi: 10.1016/j.bbi.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mayer CL, Huber BR, Peskind E. Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache. 2013;53:1523–30. doi: 10.1111/head.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 133.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–83. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 134.Huang H, He W, Tang T, Qiu M. Immunological markers for central nervous system glia. Neurosci Bull. 2023;39:379–92. doi: 10.1007/s12264-022-00938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teixeira-Santos L, Albino-Teixeira A, Pinho D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol Res. 2020;162:105280. doi: 10.1016/j.phrs.2020.105280. [DOI] [PubMed] [Google Scholar]
- 136.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell. 2018;172:500–16.e16. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu ZZ, Berta T, Ji RR. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol. 2013;8:37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leuti A, Fava M, Pellegrini N, Maccarrone M. Role of specialized pro-resolving mediators in neuropathic pain. Front Pharmacol. 2021;12:717993. doi: 10.3389/fphar.2021.717993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang YH, Tang YR, Gao X, Zhang NN, Lv QQ, Liu J, et al. Aspirin-triggered resolvin D1 ameliorates activation of the NLRP3 inflammasome via induction of autophagy in a rat model of neuropathic pain. Front Pharmacol. 2023;14:971136. doi: 10.3389/fphar.2023.971136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim PS, Fishman MA. Low-dose naltrexone for chronic pain: update and systemic review. Curr Pain Headache Rep. 2020;24:64. doi: 10.1007/s11916-020-00898-0. [DOI] [PubMed] [Google Scholar]
- 141.Vergne-Salle P, Bertin P. Chronic pain and neuroinflammation. Joint Bone Spine. 2021;88:105222. doi: 10.1016/j.jbspin.2021.105222. [DOI] [PubMed] [Google Scholar]
- 142.Yi MH, Liu YU, Liu K, Chen T, Bosco DB, Zheng J, et al. Chemogenetic manipulation of microglia inhibits neuroinflammation and neuropathic pain in mice. Brain Behav Immun. 2021;92:78–89. doi: 10.1016/j.bbi.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 144.Nijs J, Mairesse O, Neu D, Leysen L, Danneels L, Cagnie B, et al. Sleep disturbances in chronic pain: neurobiology, assessment, and treatment in physical therapist practice. Phys Ther. 2018;98:325–35. doi: 10.1093/ptj/pzy020. [DOI] [PubMed] [Google Scholar]
- 145.Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86:741–6. [PubMed] [Google Scholar]
- 146.Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth. 2019;123:637–54. doi: 10.1016/j.bja.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 147.Schwartz M, Abellanas MA, Tsitsou-Kampeli A, Suzzi S. The brain-immune ecosystem: implications for immunotherapy in defeating neurodegenerative diseases. Neuron. 2022;110:3421–4. doi: 10.1016/j.neuron.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 148.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 149.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 150.Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci. 2019;22:1771–81. doi: 10.1038/s41593-019-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 152.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–89. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 153.Zhao J, Roberts A, Wang Z, Savage J, Ji RR. Emerging role of PD-1 in the central nervous system and brain diseases. Neurosci Bull. 2021;37:1188–202. doi: 10.1007/s12264-021-00683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao J, Bang S, Furutani K, McGinnis A, Jiang C, Roberts A, et al. PD-L1/PD-1 checkpoint pathway regulates hippocampal neuronal excitability and learning and memory behavior. Neuron. 2023;111:2709–26. doi: 10.1016/j.neuron.2023.05.022. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cserep C, Posfai B, Lenart N, Fekete R, Laszlo ZI, Lele Z, et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367:528–37. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 157.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9:1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 159.Simonetti M, Hagenston AM, Vardeh D, Freitag HE, Mauceri D, Lu J, et al. Nuclear calcium signaling in spinal neurons drives a genomic program required for persistent inflammatory pain. Neuron. 2013;77:43–57. doi: 10.1016/j.neuron.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci. 2015;35:13029–42. doi: 10.1523/jneurosci.1698-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100:120–34.e6. doi: 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–5. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 164.Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain? Neuron Glia Biol. 2007;3:255–68. doi: 10.1017/s1740925x08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007;21:624–33. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–406. doi: 10.1523/jneurosci.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Echeverry S, Shi XQ, Yang M, Huang H, Wu Y, Lorenzo LE, et al. Spinal microglia are required for long-term maintenance of neuropathic pain. Pain. 2017;158:1792–801. doi: 10.1097/j.pain.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 168.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 169.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–22. doi: 10.1523/jneurosci.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Therapeut. 2003;306:624–30. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 171.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 172.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–3. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci USA. 2016;113:E3441–50. doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grace PM, Wang X, Strand KA, Baratta MV, Zhang Y, Galer EL, et al. DREADDed microglia in pain: implications for spinal inflammatory signaling in male rats. Exp Neurol. 2018;304:125–31. doi: 10.1016/j.expneurol.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Parusel S, Yi MH, Hunt CL, Wu LJ. Chemogenetic and optogenetic manipulations of microglia in chronic pain. Neurosci Bull. 2023;39:368–78. doi: 10.1007/s12264-022-00937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19:138–52. doi: 10.1038/nrn.2018.2. [DOI] [PubMed] [Google Scholar]
- 177.Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Invest. 2016;7:17–26. doi: 10.1111/jdi.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tansley S, Gu N, Guzman AU, Cai W, Wong C, Lister KC, et al. Microglia-mediated degradation of perineuronal nets promotes pain. Science. 2022;377:80–6. doi: 10.1126/science.abl6773. [DOI] [PubMed] [Google Scholar]
- 179.Tu Y, Muley MM, Beggs S, Salter MW. Microglia-independent peripheral neuropathic pain in male and female mice. Pain. 2022;163:e1129–44. doi: 10.1097/j.pain.0000000000002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27:3844–59.e6. doi: 10.1016/j.celrep.2019.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pan TT, Pan F, Gao W, Hu SS, Wang D. Involvement of macrophages and spinal microglia in osteoarthritis pain. Curr Rheumatol Rep. 2021;23:29. doi: 10.1007/s11926-021-00997-w. [DOI] [PubMed] [Google Scholar]
- 182.Ji A, Xu J. Neuropathic pain: biomolecular intervention and imaging via targeting microglia activation. Biomolecules. 2021;11:1343. doi: 10.3390/biom11091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aldskogius H, Kozlova EN. Microglia and neuropathic pain. CNS Neurol Disord Drug Targets. 2013;12:768–72. doi: 10.2174/18715273113126660168. [DOI] [PubMed] [Google Scholar]
- 184.Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–51. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–61. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–83. doi: 10.1074/jbc.m607277200. [DOI] [PubMed] [Google Scholar]
- 187.Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N, et al. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female wistar rats via P2X7 receptor and IL-18. J Neurosci. 2015;35:7950–63. doi: 10.1523/jneurosci.5250-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett. 2011;504:57–61. doi: 10.1016/j.neulet.2011.08.058. [DOI] [PubMed] [Google Scholar]
- 189.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–59. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 190.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28:2892–902. doi: 10.1523/jneurosci.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 192.Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, et al. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci. 2006;26:8680–90. doi: 10.1523/jneurosci.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–94. doi: 10.1523/jneurosci.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci. 2008;28:12775–87. doi: 10.1523/jneurosci.3512-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 196.Park CK, Lu N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1- and TNF-a-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–85. doi: 10.1523/jneurosci.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144:110–8. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gruber-Schoffnegger D, Drdla-Schutting R, Honigsperger C, Wunderbaldinger G, Gassner M, Sandkuhler J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J Neurosci. 2013;33:6540–51. doi: 10.1523/jneurosci.5087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–60. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 202.Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, et al. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 203.Clark AK, Staniland AA, Malcangio M. Fractalkine/CX3CR1 signalling in chronic pain and inflammation. Curr Pharmaceut Biotechnol. 2011;12:1707–14. doi: 10.2174/138920111798357465. [DOI] [PubMed] [Google Scholar]
- 204.Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci. 2009;29:6945–54. doi: 10.1523/jneurosci.0828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, et al. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci. 2010;30:5437–50. doi: 10.1523/jneurosci.5169-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Baumgartner R, Meder G, Briand C, Decock A, D’arcy A, Hassiepen U, et al. The crystal structure of caspase-6, a selective effector of axonal degeneration. Biochem J. 2009;423:429–39. doi: 10.1042/bj20090540. [DOI] [PubMed] [Google Scholar]
- 208.Berta T, Perrin FE, Pertin M, Tonello R, Liu YC, Chamessian A, et al. Gene expression profiling of cutaneous injured and non-injured nociceptors in SNI animal model of neuropathic pain. Sci Rep. 2017;7:9367. doi: 10.1038/s41598-017-08865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin Invest. 2014;124:1173–86. doi: 10.1172/jci72230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Obata K, Katsura H, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, et al. Roles of extracellular signal-regulated protein kinases 5 in spinal microglia and primary sensory neurons for neuropathic pain. J Neurochem. 2007;102:1569–84. doi: 10.1111/j.1471-4159.2007.04656.x. [DOI] [PubMed] [Google Scholar]
- 211.Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DL. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia. 2011;59:554–68. doi: 10.1002/glia.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tatsumi E, Yamanaka H, Kobayashi K, Yagi H, Sakagami M, Noguchi K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia. 2015;63:216–28. doi: 10.1002/glia.22745. [DOI] [PubMed] [Google Scholar]
- 213.Liu T, Gao YJ, Ji RR. Emerging role of toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–44. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234:316–29. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Masuda T, Ozono Y, Mikuriya S, Kohro Y, Tozaki-Saitoh H, Iwatsuki K, et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat Commun. 2016;7:12529. doi: 10.1038/ncomms12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev. 2010;63:222–32. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 217.Trang T, Beggs S, Salter MW. ATP receptors gate microglia signaling in neuropathic pain. Exp Neurol. 2012;234:354–61. doi: 10.1016/j.expneurol.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–28. doi: 10.1523/jneurosci.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–8. doi: 10.1523/jneurosci.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–96. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 222.Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18:595–9. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60:1529–39. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- 224.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–56. doi: 10.1523/jneurosci.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/aia.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- 227.Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419–27. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu Y, Zhou LJ, Wang J, Li D, Ren WJ, Peng J, et al. TNF-alpha differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J Neurosci. 2017;37:871–81. doi: 10.1523/jneurosci.2235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kronschlager MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkuhler J. Gliogenic LTP spreads widely in nociceptive pathways. Science. 2016;354:1144–8. doi: 10.1126/science.aah5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Xu ZZ, Liu XJ, Berta T, Park CK, Lu N, Serhan CN, et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol. 2013;74:490–5. doi: 10.1002/ana.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tang S, Jing H, Song F, Huang H, Li W, Xie G, et al. MicroRNAs in the spinal microglia serve critical roles in neuropathic pain. Mol Neurobiol. 2021;58:132–42. doi: 10.1007/s12035-020-02102-1. [DOI] [PubMed] [Google Scholar]
- 232.Tramullas M, Frances R, de la Fuente R, Velategui S, Carcelen M, Garcia R, et al. MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci Transl Med. 2018;10:eaao6299. doi: 10.1126/scitranslmed.aao6299. [DOI] [PubMed] [Google Scholar]
- 233.Tansley S, Uttam S, Urena Guzman A, Yaqubi M, Pacis A, Parisien M, et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat Commun. 2022;13:843. doi: 10.1038/s41467-022-28473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Muralidharan A, Sotocinal SG, Yousefpour N, Akkurt N, Lima LV, Tansley S, et al. Long-term male-specific chronic pain via telomere- and p53-mediated spinal cord cellular senescence. J Clin Invest. 2022;132:e151817. doi: 10.1172/jci151817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kohno K, Shirasaka R, Yoshihara K, Mikuriya S, Tanaka K, Takanami K, et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science. 2022;376:86–90. doi: 10.1126/science.abf6805. [DOI] [PubMed] [Google Scholar]
- 236.Yang J, Xie S, Zhu S, Xu ZZ. Specialized microglia resolve neuropathic pain in the spinal cord. Neurosci Bull. 2023;39:173–5. doi: 10.1007/s12264-022-00932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tang Z, Cao F, Zhang H, Tang J, Li H, Zhang Y, et al. Peripheral pain is enhanced by insulin-like growth factor 1 and its receptors in a mouse model of type 2 diabetes mellitus. J Diabetes. 2019;11:309–15. doi: 10.1111/1753-0407.12841. [DOI] [PubMed] [Google Scholar]
- 238.Romero-Sandoval EA, Horvath R, Landry RP, DeLeo JA. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain. 2009;5:25. doi: 10.1186/1744-8069-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.van den Hoogen NJ, Harding EK, Davidson CED, Trang T. Cannabinoids in chronic pain: therapeutic potential through microglia modulation. Front Neural Circ. 2021;15:816747. doi: 10.3389/fncir.2021.816747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–4. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 241.Romero-Sandoval A, Eisenach JC. Clonidine reduces hypersensitivity and alters the balance of pro- and anti-inflammatory leukocytes after local injection at the site of inflammatory neuritis. Brain Behav Immun. 2007;21:569–80. doi: 10.1016/j.bbi.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.McKelvey R, Berta T, Old E, Ji RR, Fitzgerald M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J Neurosci. 2015;35:457–66. doi: 10.1523/jneurosci.2315-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jia X, Li Z, Shen X, Zhang Y, Zhang L, Zhang L. High-intensity swimming alleviates nociception and neuroinflammation in a mouse model of chronic post-ischemia pain by activating the resolvin E1-chemerin receptor 23 axis in the spinal cord. Neural Regen Res. 2023;18:2535–44. doi: 10.4103/1673-5374.371373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fernandez-Zafra T, Gao T, Jurczak A, Sandor K, Hore Z, Agalave NM, et al. Exploring the transcriptome of resident spinal microglia after collagen antibody-induced arthritis. Pain. 2019;160:224–36. doi: 10.1097/j.pain.0000000000001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018;159:1752–63. doi: 10.1097/j.pain.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 247.Saika F, Matsuzaki S, Kobayashi D, Ideguchi Y, Nakamura TY, Kishioka S, et al. Chemogenetic regulation of CX3CR1-expressing microglia using Gi-DREADD exerts sex-dependent anti-allodynic effects in mouse models of neuropathic pain. Front Pharmacol. 2020;11:925. doi: 10.3389/fphar.2020.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yao Y, Echeverry S, Shi XQ, Yang M, Yang QZ, Wang GY, et al. Dynamics of spinal microglia repopulation following an acute depletion. Sci Rep. 2016;6:22839. doi: 10.1038/srep22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Luo X, Fitzsimmons B, Mohan A, Zhang L, Terrando N, Kordasiewicz H, et al. Intrathecal administration of antisense oligonucleotide against p38alpha but not p38beta MAP kinase isoform reduces neuropathic and postoperative pain and TLR4-induced pain in male mice. Brain Behav Immun. 2018;72:34–44. doi: 10.1016/j.bbi.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Paige C, Maruthy GB, Mejia G, Dussor G, Price T. Spinal inhibition of P2XR or p38 signaling disrupts hyperalgesic priming in male, but not female, mice. Neuroscience. 2018;385:133–42. doi: 10.1016/j.neuroscience.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chen G, Luo X, Qadri MY, Berta T, Ji RR. Sex-dependent glial signaling in pathological pain: distinct roles of spinal microglia and astrocytes. Neurosci Bull. 2018;34:98–108. doi: 10.1007/s12264-017-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Posillico CK, Terasaki LS, Bilbo SD, Schwarz JM. Examination of sex and minocycline treatment on acute morphine-induced analgesia and inflammatory gene expression along the pain pathway in Sprague-Dawley rats. Biol Sex Differ. 2015;6:33. doi: 10.1186/s13293-015-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Moller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, et al. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia. 2016;64:1788–94. doi: 10.1002/glia.23007. [DOI] [PubMed] [Google Scholar]
- 254.Berta T, Qadri YJ, Chen G, Ji RR. Microglial signaling in chronic pain with a special focus on caspase 6, p38 MAP kinase, and sex dependence. J Dent Res. 2016;95:1124–31. doi: 10.1177/0022034516653604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–82. doi: 10.1523/jneurosci.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sorge RE, Lacroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, et al. Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–4. doi: 10.1523/jneurosci.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–15. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hanani M, Spray DC. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci. 2020;21:485–98. doi: 10.1038/s41583-020-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McGinnis A, Ji RR. The similar and distinct roles of satellite glial cells and spinal astrocytes in neuropathic pain. Cells. 2023;12:965. doi: 10.3390/cells12060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med. 2022;14:eabj9954. doi: 10.1126/scitranslmed.abj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kavelaars A, Heijnen CJ. Immune regulation of pain: friend and foe. Sci Transl Med. 2021;13:eabj7152. doi: 10.1126/scitranslmed.abj7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lu HJ, Gao YJ. Astrocytes in chronic pain: cellular and molecular mechanisms. Neurosci Bull. 2023;39:425–39. doi: 10.1007/s12264-022-00961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wu Y, Yang Z, Cheng K, Bi H, Chen J. Small molecule-based immunomodulators for cancer therapy. Acta Pharm Sin B. 2022;12:4287–308. doi: 10.1016/j.apsb.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 265.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med. 2016;22:135–7. doi: 10.1038/nm.4022. [DOI] [PubMed] [Google Scholar]
- 267.Zhao J, Huh Y, Bortsov A, Diatchenko L, Ji RR. Immunotherapies in chronic pain through modulation of neuroimmune interactions. Pharmacol Ther. 2023;248:108476. doi: 10.1016/j.pharmthera.2023.108476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wang K, Donnelly CR, Jiang C, Liao Y, Luo X, Tao X, et al. STING suppresses bone cancer pain via immune and neuronal modulation. Nat Commun. 2021;12:4558. doi: 10.1038/s41467-021-24867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Donnelly CR, Jiang C, Andriessen AS, Wang K, Wang Z, Ding H, et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature. 2021;591:275–80. doi: 10.1038/s41586-020-03151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kalpachidou T, Riehl L, Schopf CL, Ucar B, Kress M. Proinflammatory cytokines and their receptors as druggable targets to alleviate pathological pain. Pain. 2022;163(1 Suppl):S79–98. doi: 10.1097/j.pain.0000000000002737. [DOI] [PubMed] [Google Scholar]
- 271.Guo W, Imai S, Yang JL, Zou S, Watanabe M, Chu YX, et al. In vivo immune interactions of multipotent stromal cells underlie their long-lasting pain-relieving effect. Sci Rep. 2017;7:10107. doi: 10.1038/s41598-017-10251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Buchheit T, Huh Y, Maixner W, Cheng J, Ji RR. Neuroimmune modulation of pain and regenerative pain medicine. J Clin Invest. 2020;130:2164–76. doi: 10.1172/jci134439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Buchheit T, Huh Y, Breglio A, Bang S, Xu J, Matsuoka Y, et al. Intrathecal administration of conditioned serum from different species resolves chemotherapy-induced neuropathic pain in mice via secretory exosomes. Brain Behav Immun. 2023;111:298–311. doi: 10.1016/j.bbi.2023.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Shiue SJ, Rau RH, Shiue HS, Hung YW, Li ZX, Yang KD, et al. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain. 2019;160:210–23. doi: 10.1097/j.pain.0000000000001395. [DOI] [PubMed] [Google Scholar]