Abstract
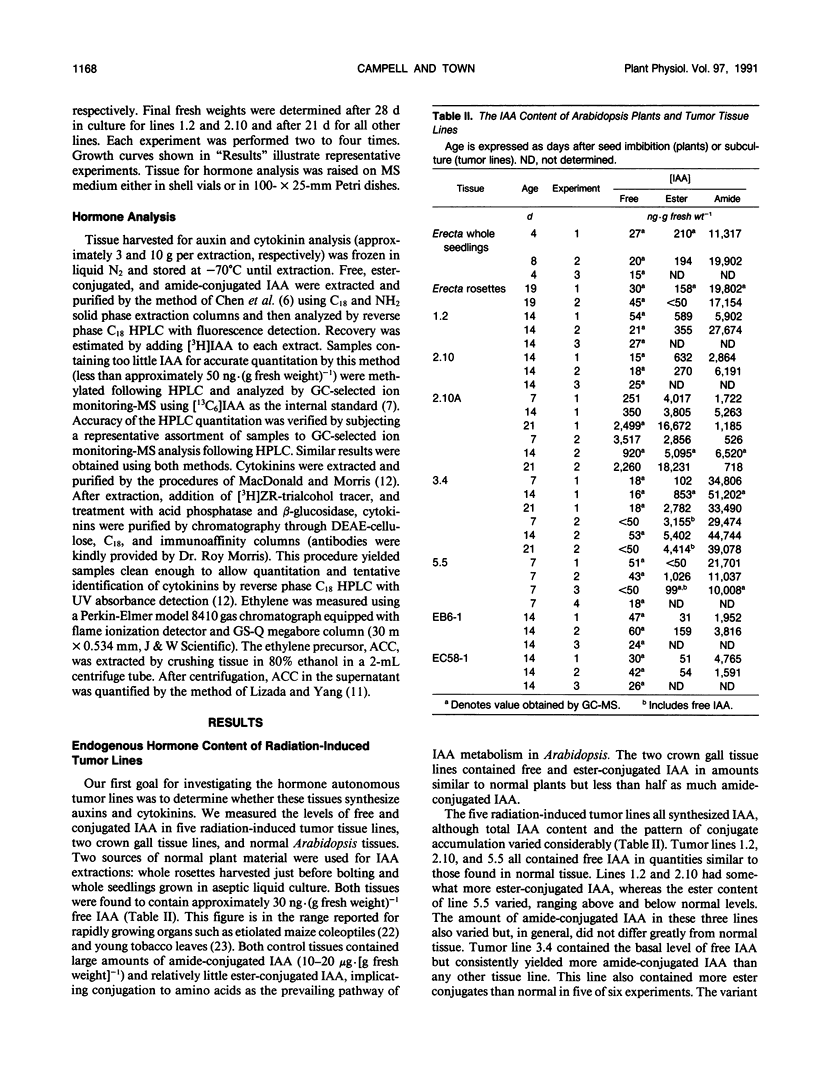
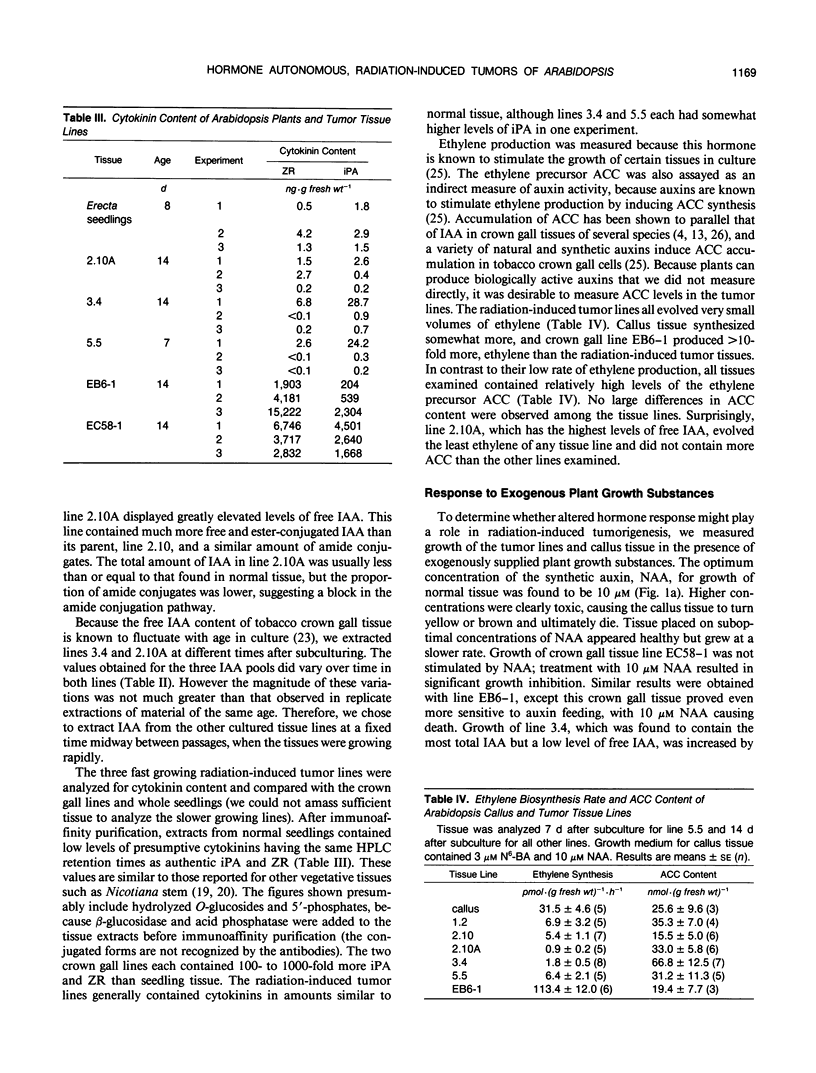
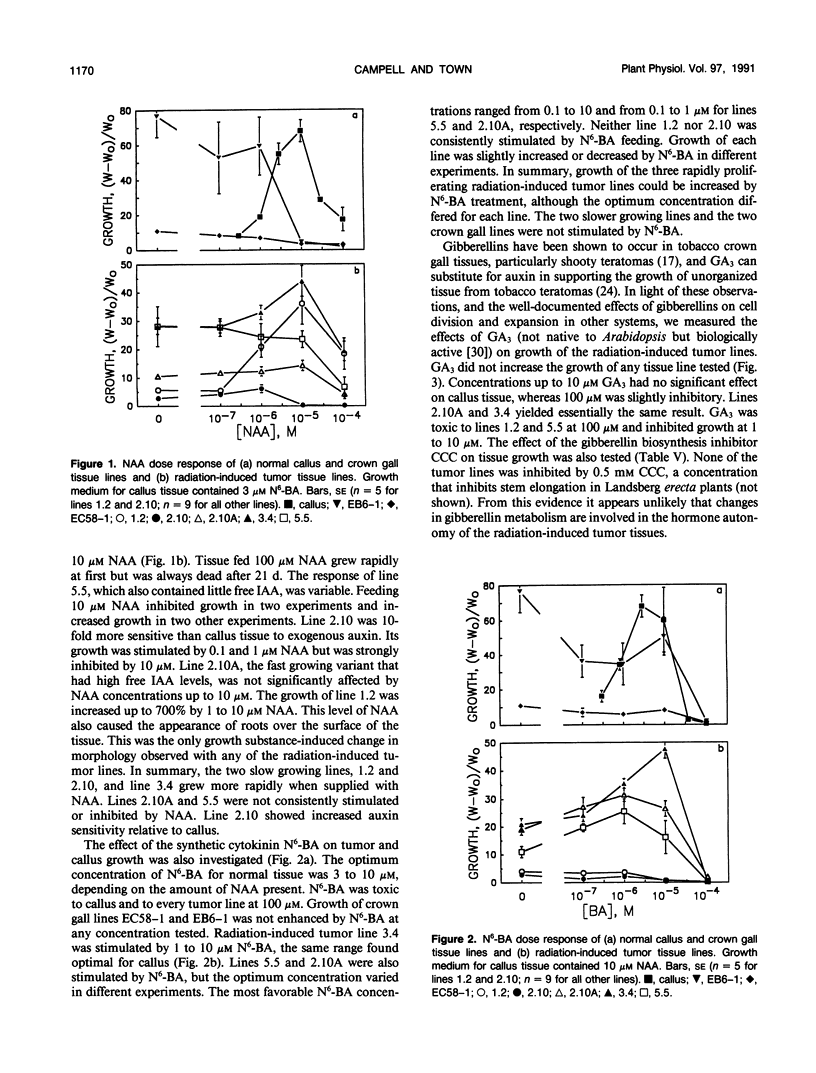
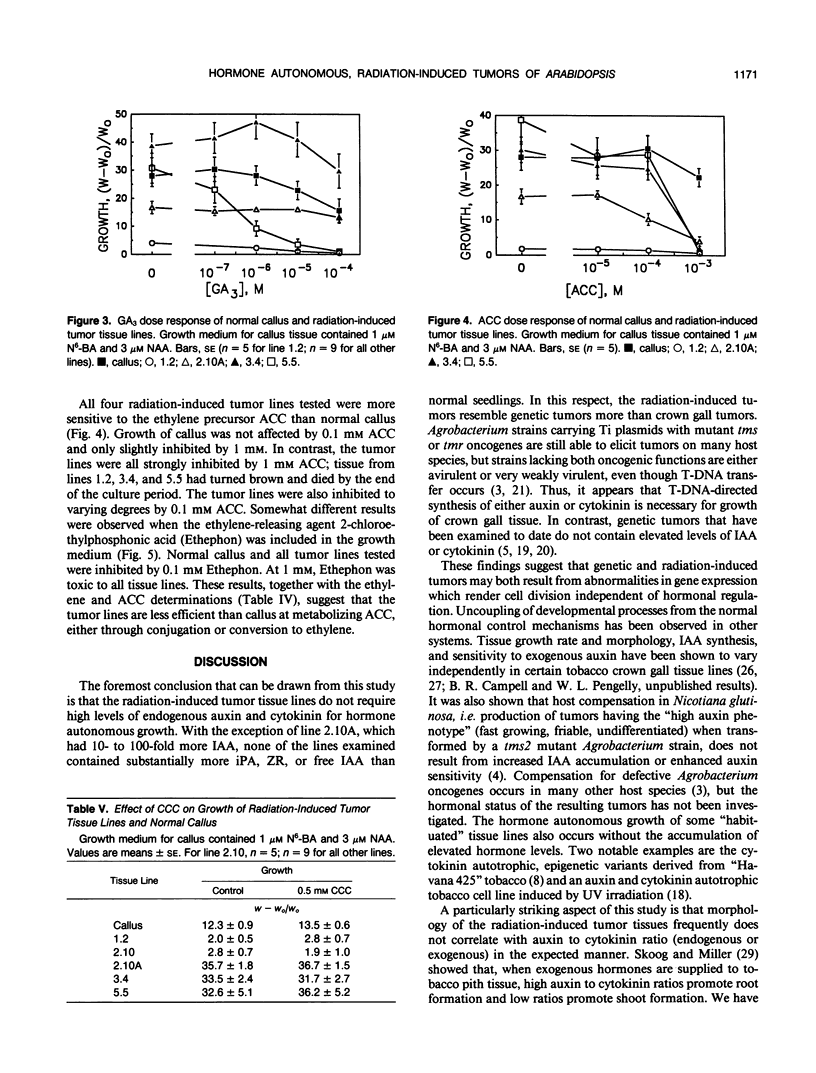
γ-Radiation-induced tumors of Arabidopsis thaliana L. have been produced as a novel approach to isolation of genes that regulate plant development. Tumors excised from irradiated plants are hormone autonomous in culture and have been maintained on hormone-free medium for up to 4 years. Five tumor tissue lines having different morphologies and growth rates were analyzed for auxin, cytokinin, and 1-aminocyclopropane-1-carboxylic acid (ACC) content, ethylene production, and response to exogenous growth regulators. Normal tissues and two crown gall tissue lines were analyzed for comparison. Rosettes and whole seedlings each contained approximately 30 nanograms· (gram fresh weight)−1 free indoleacetic acid (IAA), 150 nanograms· (gram fresh weight)−1 ester-conjugated IAA, and 10 to 20 micrograms· (gram fresh weight)−1 amide-conjugated IAA. The crown gall lines contained similar amounts of free and ester-conjugated IAA but less amide conjugates. Whereas three of the radiation-induced tumor lines had IAA profiles similar to normal tissues, one line had 10- to 100-fold more free IAA and three- to 10-fold less amide-conjugated IAA. The fifth line had normal free IAA levels but more conjugated IAA than control tissues. Whole seedlings contained approximately 2 nanograms· (gram fresh weight)−1 of both zeatin riboside and isopentenyladenosine. The crown gall lines had 100- to 1000-fold higher levels of each cytokinin. In contrast, the three radiation-induced tumor lines analyzed contained cytokinin levels similar to the control tissue. The radiation-induced tumor tissues produced very little ethylene, although each contained relatively high levels of ACC. Normal callus contained similar amounts of ACC but produced several times more ethylene than the radiation-induced tumor lines. Each of the radiation-induced tumor tissues displayed a unique set of responses to exogenously supplied growth regulators. Only one tumor line showed the same response as normal callus to both auxin and cytokinin feeding. In some cases, one or more tumor lines showed increased sensitivity to certain growth substances. In other cases, growth regulator feeding had no significant effect on tumor tissue growth. Morphology of the radiation-induced tumor tissues generally did not correlate with auxin to cytokinin ratio in the expected manner. The results suggest that a different primary genetic event led to the formation of each tumor and that growth and differentiation in the tumor tissue lines are uncoupled from the normal hormonal controls.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campell B. R., Su L. Y., Pengelly W. L. Compensation for a Mutated Auxin Biosynthesis Gene of Agrobacterium Ti Plasmid A66 in Nicotiana glutinosa Does Not Result from Increased Auxin Accumulation. Plant Physiol. 1989 Apr;89(4):1337–1340. doi: 10.1104/pp.89.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. H., Miller A. N., Patterson G. W., Cohen J. D. A Rapid and Simple Procedure for Purification of Indole-3-Acetic Acid Prior to GC-SIM-MS Analysis. Plant Physiol. 1988 Mar;86(3):822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Baldi B. G., Slovin J. P. C(6)-[benzene ring]-indole-3-acetic Acid: a new internal standard for quantitative mass spectral analysis of indole-3-acetic Acid in plants. Plant Physiol. 1986 Jan;80(1):14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Morrow R. C., Tibbitts T. W. Evidence for involvement of phytochrome in tumor development on plants. Plant Physiol. 1988;88:1110–1114. doi: 10.1104/pp.88.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S. K., Palni L. M., Parker C. W. Dynamics of Endogenous Cytokinins during the Growth Cycle of a Hormone-Autotrophic Genetic Tumor Line of Tobacco. Plant Physiol. 1990 Nov;94(3):1084–1089. doi: 10.1104/pp.94.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelly W. L., Bandurski R. S., Schulze A. Validation of a radioimmunoassay for indole-3-acetic Acid using gas chromatography-selected ion monitoring-mass spectrometry. Plant Physiol. 1981 Jul;68(1):96–98. doi: 10.1104/pp.68.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelly W. L., Meins F., Jr The relationship of indole-3-acetic acid content and growth of crown-gall tumor tissues of tobacco in culture. Differentiation. 1982;21(1):27–31. doi: 10.1111/j.1432-0436.1982.tb01190.x. [DOI] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Talon M., Koornneef M., Zeevaart J. A. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7983–7987. doi: 10.1073/pnas.87.20.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]


