Abstract
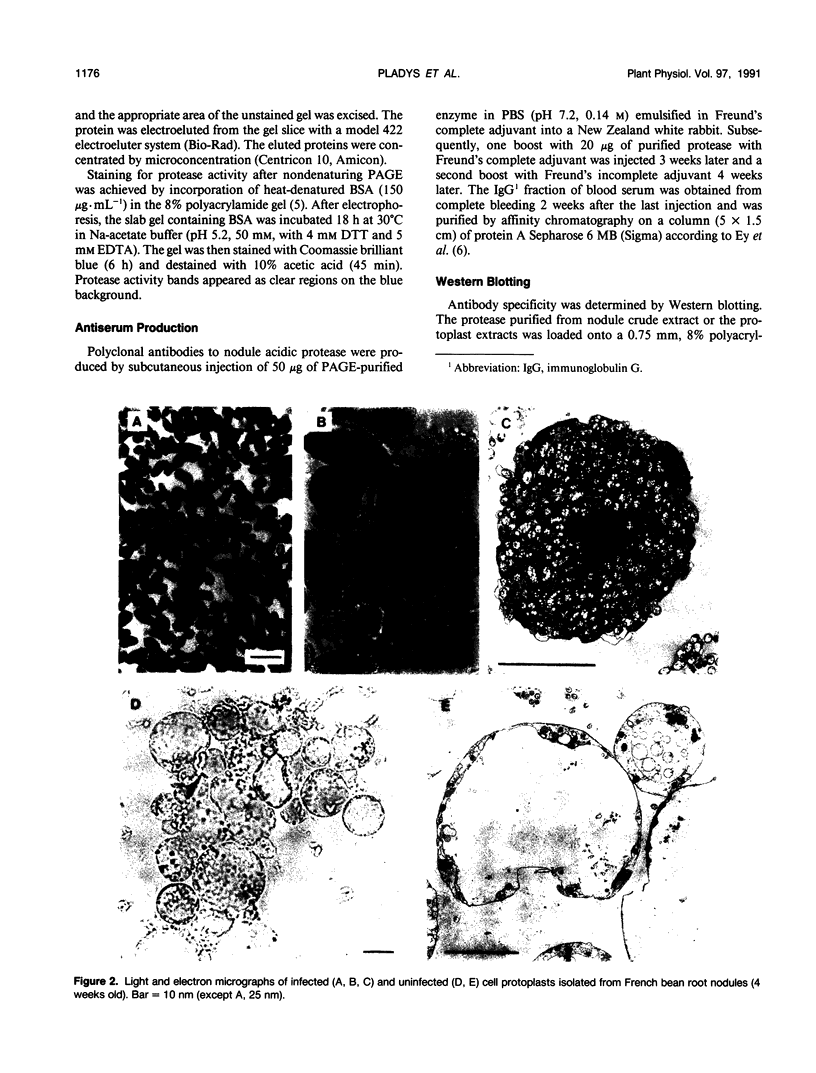
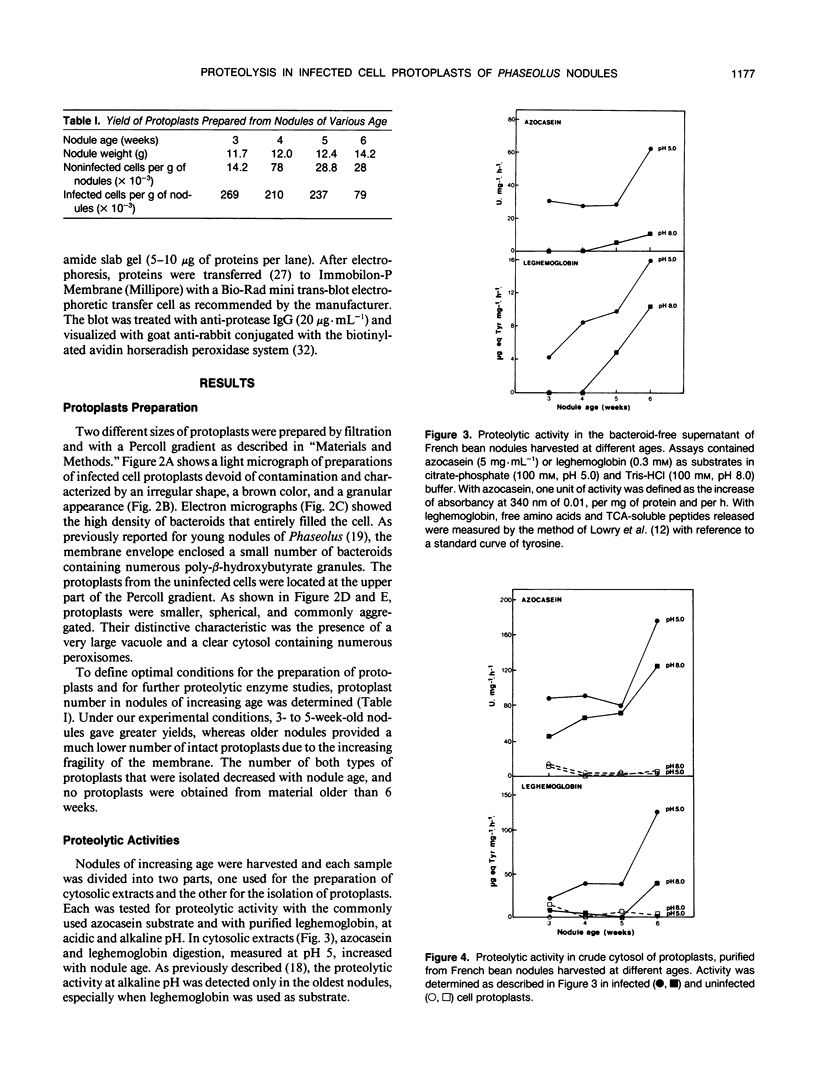
Protoplasts from infected and uninfected cells were isolated from the central nitrogen fixing tissue of French bean (Phaseolus vulgaris L. cv Contender) root nodules. Successive filtrations allowed the separation of the infected cells, whereas the small uninfected cells were isolated on a discontinuous Percoll gradient. Higher yields of intact protoplasts were obtained from young (4-week-old) nodules whereas no protoplasts could be isolated from the oldest nodules. When proteolysis was determined in the cytosolic fraction of both infected and uninfected cells, at pH 5.0 and 8.0, with leghemoglobin or azocasein as substrate, activity was present only in infected cell protoplasts and increased with nodule age. A protease with an acidic pH optimum, mainly responsible for this increasing activity, was highly purified from senescing nodules by electro-elution after nondenaturing polyacrylamide gel electrophoresis and used to produce polyclonal antibodies. Western blots of nodule protein separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with purified anti-protease immunoglobulin G showed the molecular mass of the protease to be 58 kilodaltons. Blots also confirmed that protease protein was located in infected cell protoplasts only, regardless of nodule age.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewin N. J., Robertson J. G., Wood E. A., Wells B., Larkins A. P., Galfre G., Butcher G. W. Monoclonal antibodies to antigens in the peribacteroid membrane from Rhizobium-induced root nodules of pea cross-react with plasma membranes and Golgi bodies. EMBO J. 1985 Mar;4(3):605–611. doi: 10.1002/j.1460-2075.1985.tb03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. L., Yet M. G., Wold F. Substrate-containing gel electrophoresis: sensitive detection of amylolytic, nucleolytic, and proteolytic enzymes. Anal Biochem. 1982 May 1;122(1):164–172. doi: 10.1016/0003-2697(82)90266-4. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Hanks J. F., Schubert K., Tolbert N. E. Isolation and characterization of infected and uninfected cells from soybean nodules : role of uninfected cells in ureide synthesis. Plant Physiol. 1983 Apr;71(4):869–873. doi: 10.1104/pp.71.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manen J. F., Simon P., Van Slooten J. C., Osterås M., Frutiger S., Hughes G. J. A nodulin specifically expressed in senescent nodules of winged bean is a protease inhibitor. Plant Cell. 1991 Mar;3(3):259–270. doi: 10.1105/tpc.3.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer N. E., Torres C. M., Wagner F. W. Proteolytic Activity in Soybean Root Nodules : Activity in Host Cell Cytosol and Bacteroids throughout Physiological Development and Senescence. Plant Physiol. 1983 Apr;71(4):797–802. doi: 10.1104/pp.71.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A., Herrada G., Rigaud J. Lipid peroxidation in peribacteroid membranes from French-bean nodules. Plant Physiol. 1991 Jul;96(3):826–830. doi: 10.1104/pp.96.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]