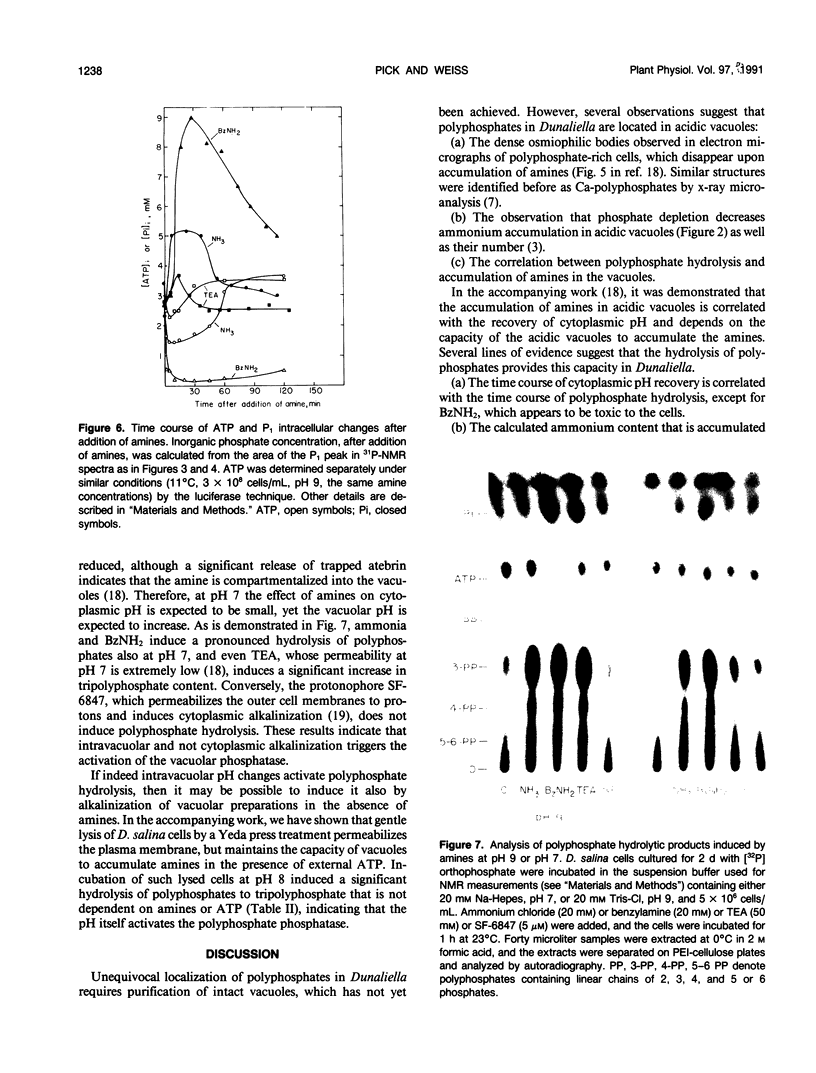
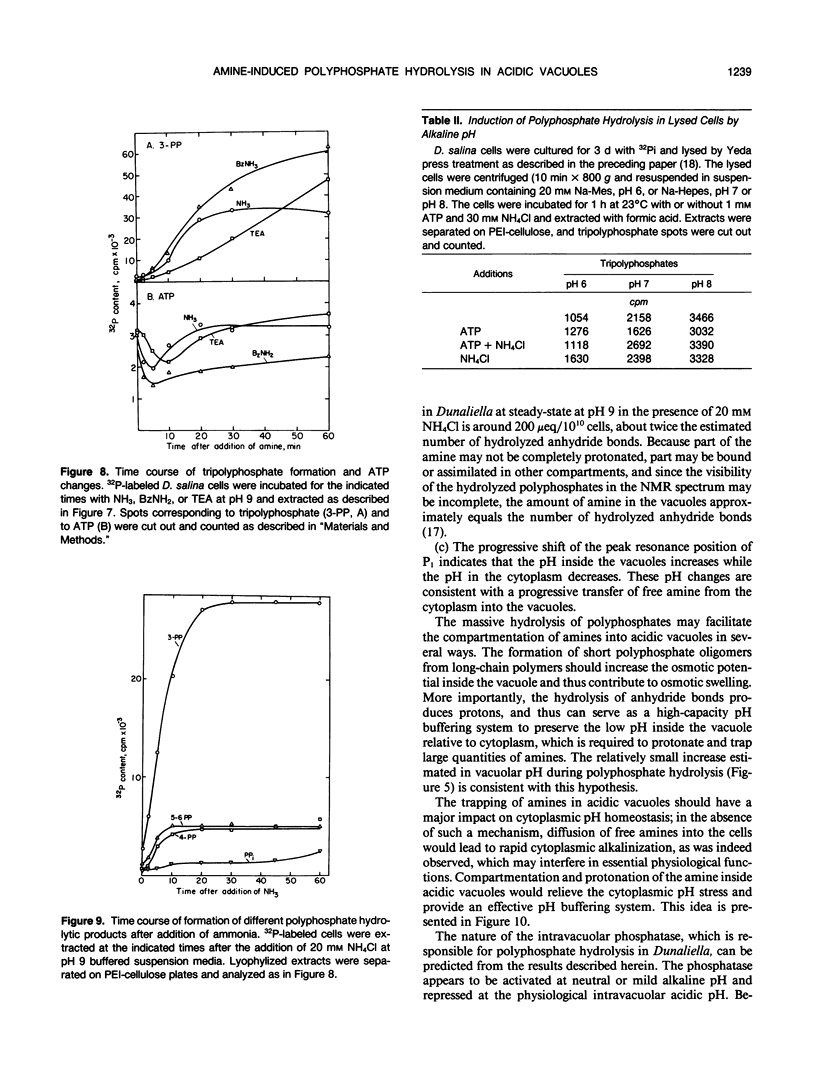
Abstract
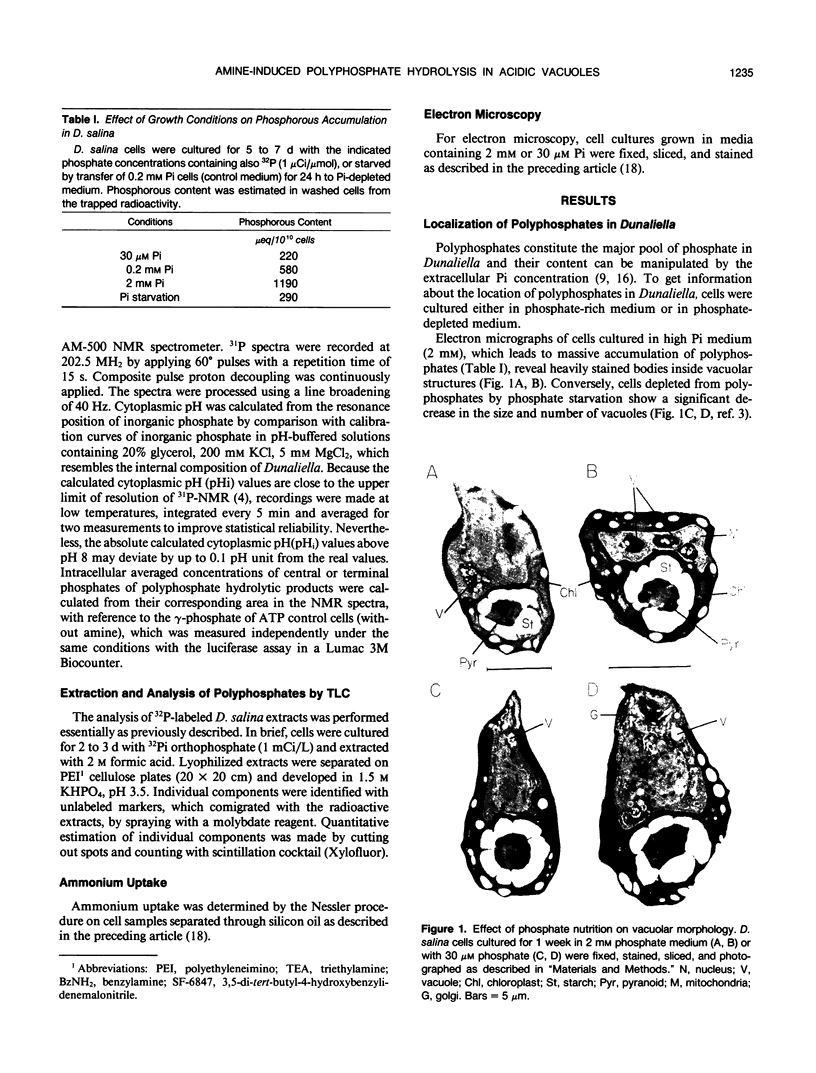
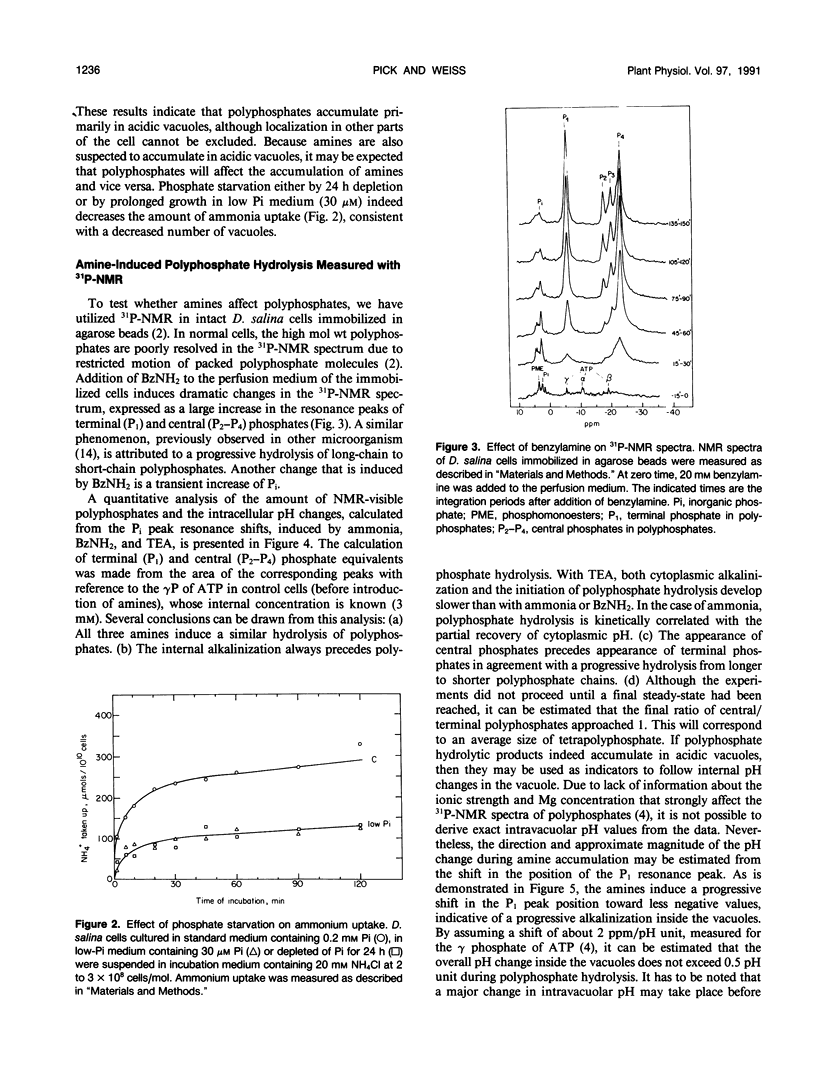
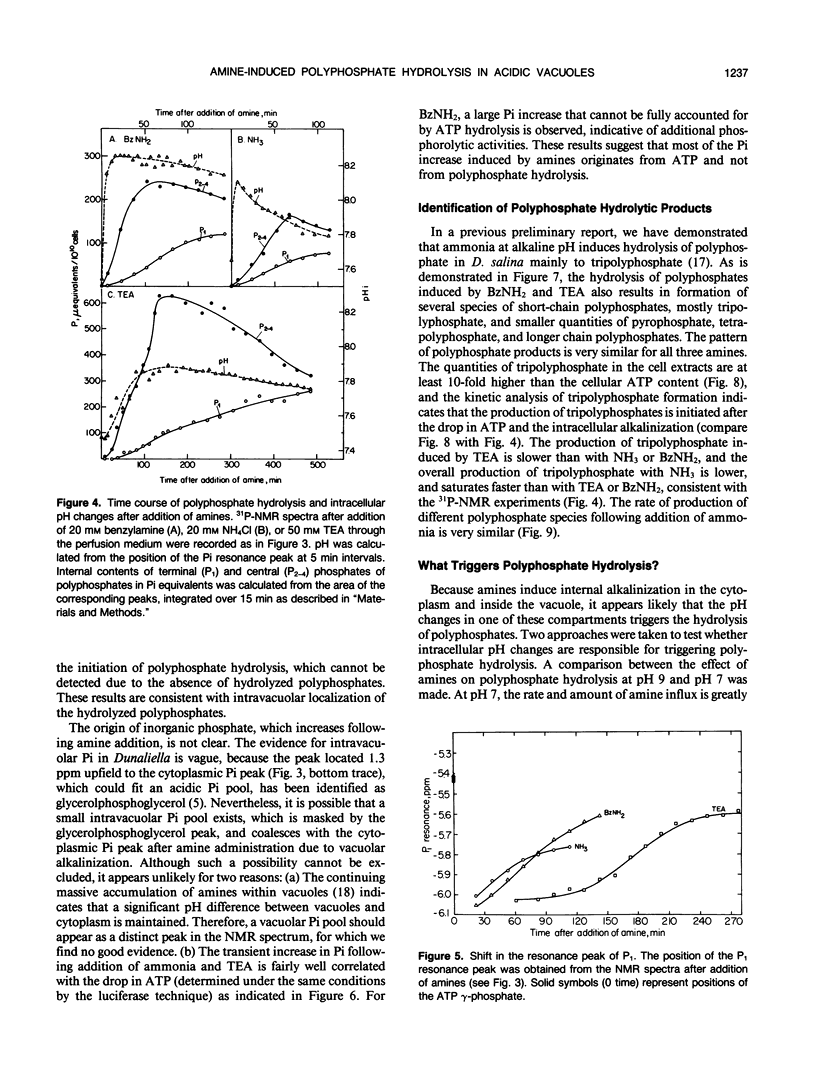
The location and mobilization of polyphosphates in response to an amine-induced alkaline stress were studied in the halotolerant alga Dunaliella salina. The following observations suggest that polyphosphates accumulate in acidic vacuoles: (a) Accumulation of large amounts of polyphosphates is manifested as intravacuolar dense osmiophilic bodies in electron micrographs. (b) Uptake of amines into the vacuoles induces massive hydrolysis of polyphosphates, demonstrated by in vivo 31P-nuclear magnetic resonance, and by analysis of hydrolytic products on thin layer chromatograms. The analysis indicates that: (a) Polyphosphate hydrolysis is kinetically correlated with amine accumulation and with the recovery of cytoplasmic pH. (b) The major hydrolytic product is tripolyphosphate. (c) The peak position of the tripolyphosphate terminal phosphate in nuclear magnetic resonance spectra is progressively shifted as the cells recover, indicating that the pH inside the vacuoles increases while the pH in the cytoplasm decreases. (d) In lysed cell preparations, in which vacuoles become exposed to the external pH, mild alkalinization in the absence of amines induces polyphosphate hydrolysis to tripolyphosphates. It is suggested that amine accumulation within vacuoles activates a specific phosphatase, which hydrolyzes long-chain polyphosphates to tripolyphosphates. The hydrolysis increases the capacity of the vacuoles to sequester amines from the cytoplasm probably by releasing protons required to buffer the amine, and leads to recovery of cytoplasmic pH. Thus, polyphosphate hydrolysis provides a high-capacity buffering system that sustains amine compartmentation into vacuoles and protects cytoplasmic pH.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn K., Kornberg A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J Biol Chem. 1990 Jul 15;265(20):11734–11739. [PubMed] [Google Scholar]
- Bental M., Pick U., Avron M., Degani H. Metabolic studies with NMR spectroscopy of the alga Dunaliella salina trapped within agarose beads. Eur J Biochem. 1990 Feb 22;188(1):111–116. doi: 10.1111/j.1432-1033.1990.tb15377.x. [DOI] [PubMed] [Google Scholar]
- Bental M., Pick U., Avron M., Degani H. Polyphosphate metabolism in the alga Dunaliella salina studied by 31P-NMR. Biochim Biophys Acta. 1991 Mar 19;1092(1):21–28. doi: 10.1016/0167-4889(91)90173-u. [DOI] [PubMed] [Google Scholar]
- Greenfield N. J., Hussain M., Lenard J. Effects of growth state and amines on cytoplasmic and vacuolar pH, phosphate and polyphosphate levels in Saccharomyces cerevisiae: a 31P-nuclear magnetic resonance study. Biochim Biophys Acta. 1987 Dec 7;926(3):205–214. doi: 10.1016/0304-4165(87)90205-4. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. Depletion and replenishment of the inorganic polyphosphate pool in Neurospora crassa. J Bacteriol. 1962 May;83:1047–1057. doi: 10.1128/jb.83.5.1047-1057.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG A., KORNBERG S. R., SIMMS E. S. Metaphosphate synthesis by an enzyme from Escherichia coli. Biochim Biophys Acta. 1956 Apr;20(1):215–227. doi: 10.1016/0006-3002(56)90280-3. [DOI] [PubMed] [Google Scholar]
- Kulaev I. S., Vagabov V. M. Polyphosphate metabolism in micro-organisms. Adv Microb Physiol. 1983;24:83–171. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- LISS E., LANGEN P. [On a high-molecular weight polyphosphate of yeast]. Biochem Z. 1960;333:193–201. [PubMed] [Google Scholar]
- Ludwig J. R., 2nd, Oliver S. G., McLaughlin C. S. The effect of amino acids on growth and phosphate metabolism in a prototrophic yeast strain. Biochem Biophys Res Commun. 1977 Nov 7;79(1):16–23. doi: 10.1016/0006-291x(77)90054-7. [DOI] [PubMed] [Google Scholar]
- Lusby E. W., Jr, McLaughlin C. S. The metabolic properties of acid soluble polyphosphates in Saccharomyces cerevisiae. Mol Gen Genet. 1980 Apr;178(1):69–76. doi: 10.1007/BF00267214. [DOI] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Pick U., Ben-Amotz A., Karni L., Seebergts C. J., Avron M. Partial Characterization of K and Ca Uptake Systems in the Halotolerant Alga Dunaliella salina. Plant Physiol. 1986 Jul;81(3):875–881. doi: 10.1104/pp.81.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
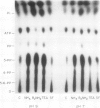
- Pick U., Bental M., Chitlaru E., Weiss M. Polyphosphate-hydrolysis--a protective mechanism against alkaline stress? FEBS Lett. 1990 Nov 12;274(1-2):15–18. doi: 10.1016/0014-5793(90)81318-i. [DOI] [PubMed] [Google Scholar]
- Pick U., Zeelon O., Weiss M. Amine Accumulation in Acidic Vacuoles Protects the Halotolerant Alga Dunaliella salina Against Alkaline Stress. Plant Physiol. 1991 Nov;97(3):1226–1233. doi: 10.1104/pp.97.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Clark J. E. Biological aspects of inorganic polyphosphates. Annu Rev Biochem. 1988;57:235–260. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]