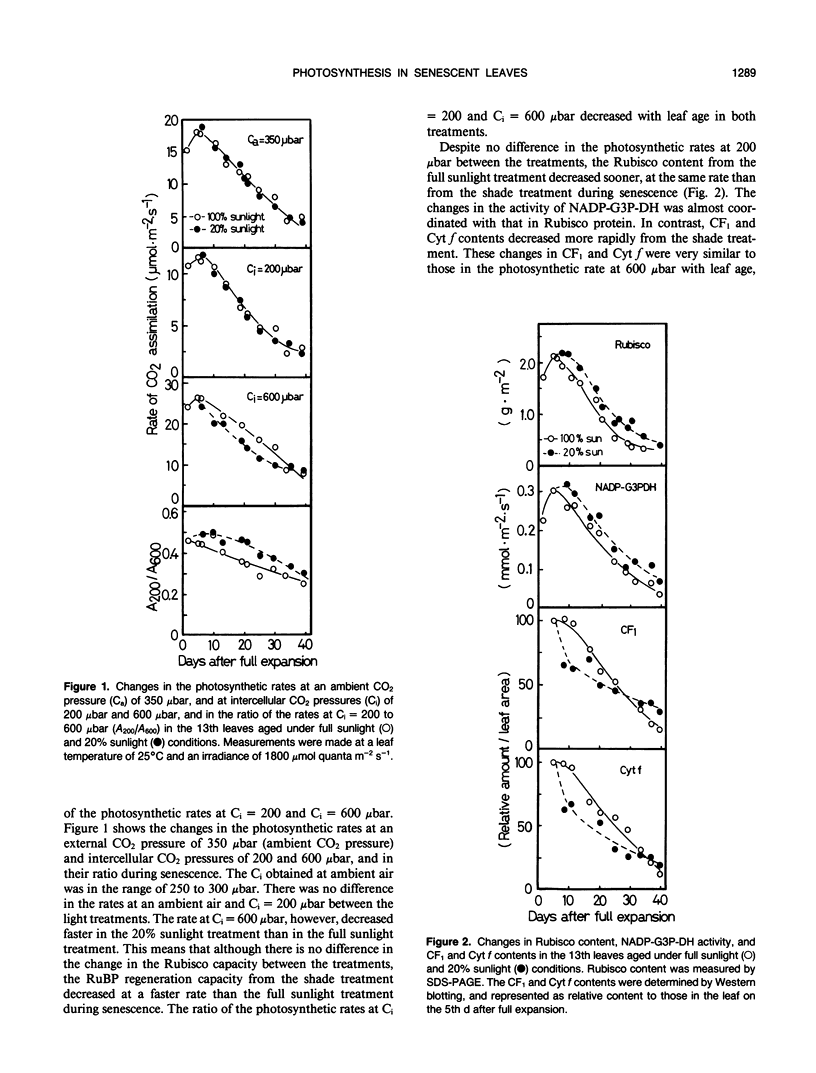
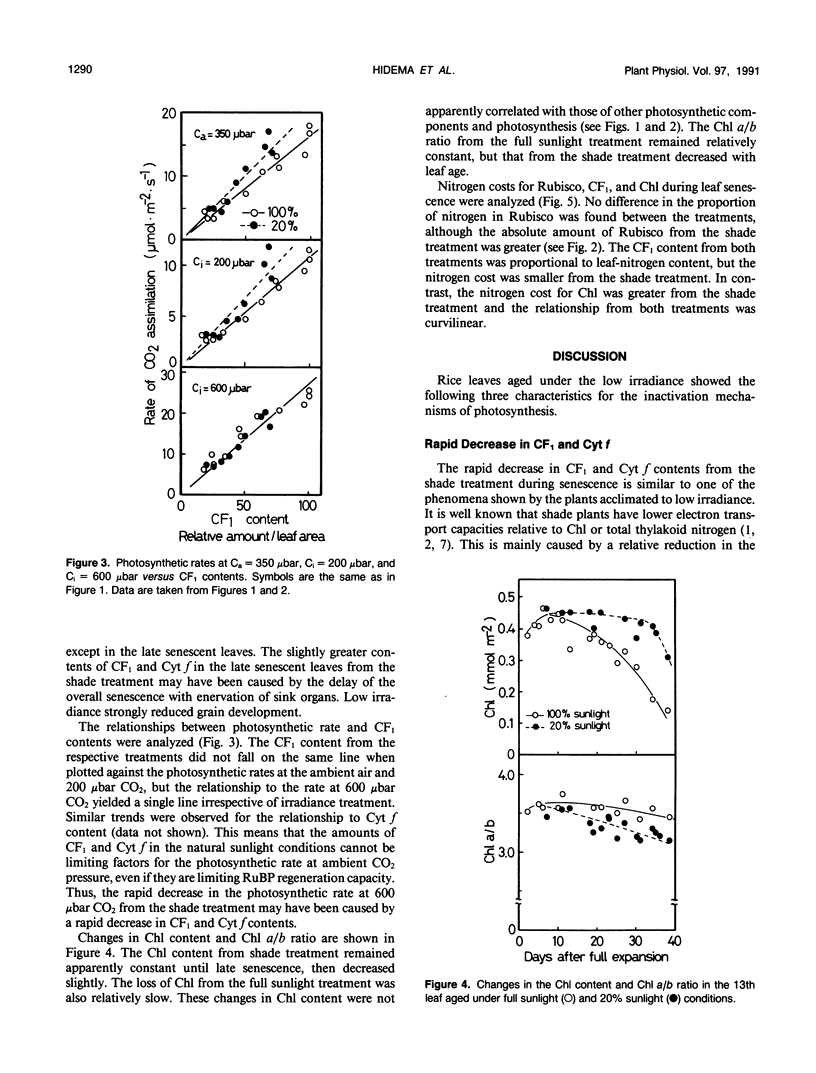
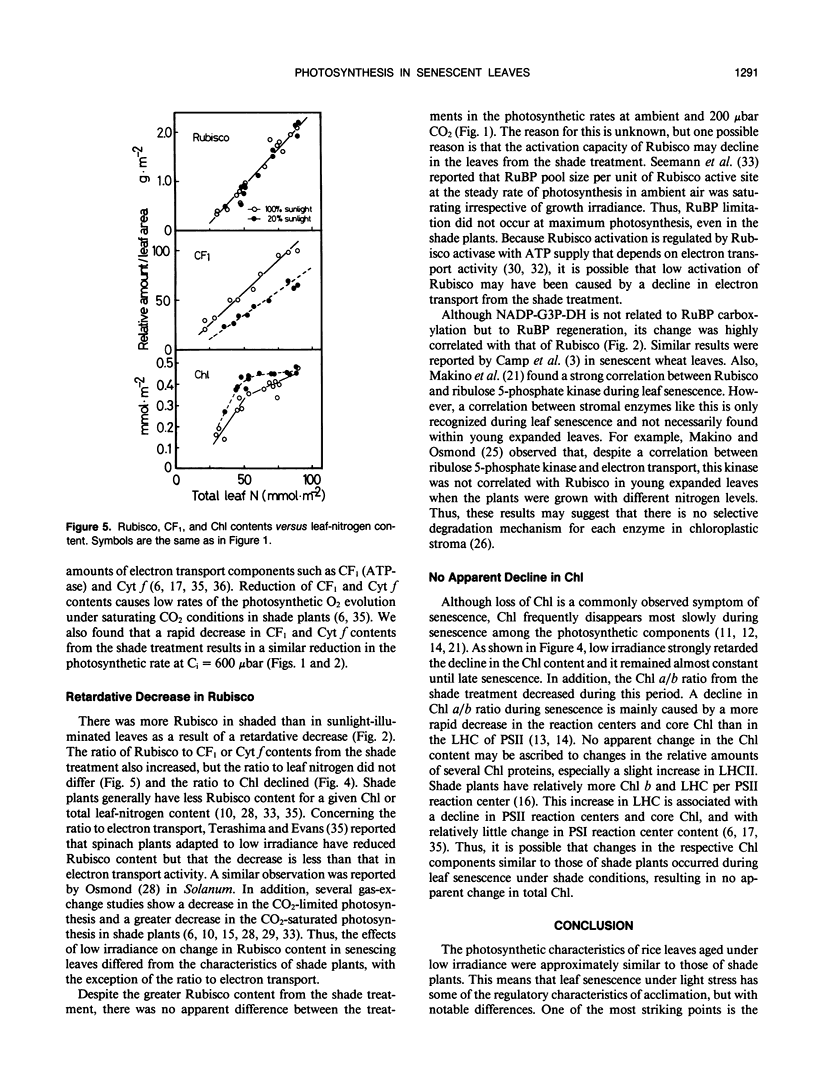
Abstract
Effects of irradiance on photosynthetic characteristics were examined in senescent leaves of rice (Oryza sativa L.). Two irradiance treatments (100 and 20% natural sunlight) were imposed after the full expansion of the 13th leaf through senescence. The photosynthetic rate was measured as a function of intercellular CO2 pressure with a gas-exchange system. The amounts of cytochrome f, coupling factor 1, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), and chlorophyll were determined. The coupling factor 1 and cytochrome f contents decreased rapidly during senescence, and their rates of decrease were much faster from the 20% sunlight treatment than from the full sunlight treatment. These changes were well correlated with those in the photosynthetic rate at CO2 pressure = 600 microbars, but not with those under the ambient air condition (350 microbars CO2) and 200 microbars CO2. This suggested that the amounts of coupling factor 1 and cytochrome f from the full sunlight treatment cannot be limiting factors for the photosynthetic rate at ambient air conditions. The Rubisco content also decreased during senescence, but its decrease from the 20% sunlight treatment was appreciably retarded. However, this difference was not reflected in the photosynthetic rates at the ambient and 200 microbars CO2. This implied that in vivo Rubisco activity may be regulated in the senescent leaves from the 20% sunlight treatment. The chlorophyll content decreased most slowly. In the 20% sunlight treatment, it remained apparently constant with a decline in chlorophyll a/b ratio. These photosynthetic characteristics of the senescent rice leaves under low irradiance were discussed in relation to acclimation of shade plants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camp P. J., Huber S. C., Burke J. J., Moreland D. E. Biochemical Changes that Occur during Senescence of Wheat Leaves : I. Basis for the Reduction of Photosynthesis. Plant Physiol. 1982 Dec;70(6):1641–1646. doi: 10.1104/pp.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. J., Maclean D. J., Scott K. J. Rate-Limiting Steps of Electron Transport in Chloroplasts during Ontogeny and Senescence of Barley. Plant Physiol. 1983 Jul;72(3):795–801. doi: 10.1104/pp.72.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A., Mae T., Ohira K. Photosynthesis and Ribulose 1,5-Bisphosphate Carboxylase in Rice Leaves: Changes in Photosynthesis and Enzymes Involved in Carbon Assimilation from Leaf Development through Senescence. Plant Physiol. 1983 Dec;73(4):1002–1007. doi: 10.1104/pp.73.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A., Osmond B. Effects of Nitrogen Nutrition on Nitrogen Partitioning between Chloroplasts and Mitochondria in Pea and Wheat. Plant Physiol. 1991 Jun;96(2):355–362. doi: 10.1104/pp.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadai K., Mae T., Makino A., Ojima K. Characteristics of ribulose-1,5-bisphosphate carboxylase/oxygenase degradation by lysates of mechanically isolated chloroplasts from wheat leaves. Plant Physiol. 1990 Apr;92(4):1215–1219. doi: 10.1104/pp.92.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. F. A Model Describing the Regulation of Ribulose-1,5-Bisphosphate Carboxylase, Electron Transport, and Triose Phosphate Use in Response to Light Intensity and CO(2) in C(3) Plants. Plant Physiol. 1990 Dec;94(4):1728–1734. doi: 10.1104/pp.94.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D., Wang J., Osmond C. B. Environmental effects on photosynthesis, nitrogen-use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiol. 1987 Jul;84(3):796–802. doi: 10.1104/pp.84.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]


