Abstract
A well-synchronized circadian system is a manifestation of an individual's health. A gradual weakening of the circadian timing function characterizes aging. Regular exercise has been suggested as a modality to improve many detrimental changes associated with aging. Therefore, we aim to examine the benefits and risks of lifelong endurance exercise on age-dependent changes in the circadian time-keeping function, the performance of the muscular system and health status. The study protocol has a comparative cross-sectional design, including groups of senior (65 to 75 years old, n=16) and young (20-30 years old, n=16) endurance runners and triathletes. Age-matched groups of young and elderly sedentary men are included as controls. The circadian function is evaluated mainly by measurement of urinary 6-sulphatoxymelatonin, a metabolite of the hormone melatonin shown to participate in the modulation of sleep cycles. The 6-sulphatoxymelatonin will be assessed in urine samples collected upon awakening in the morning and in the late evening, as a marker of melatonin production. In addition, sleep/activity rhythms and sleep quality will be measured by wrist actigraphy. Performance of the muscular system will be assessed by examination of muscular strength and quantifying of gene expression in the skeletal muscle tissue samples. Health status and age-induced reduction in immune function are to be analysed via the balance of pro- and anti-inflammatory immune markers in the plasma and skeletal muscle, body composition, bone density and physical fitness.
Key Words: circadian, aging, exercise, sports medicine, molecular biology, sleep/wake rhythm
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Parameters related to physical performance and fitness vary across a day and exhibit distinct circadian rhythms. Most performance-related parameters, from maximum strength and power production,1,2,3,4 to maximum fat oxidation during longer endurance loading,5 peak in the afternoon. A molecular background for these rhythmic changes is at least partly formed by core-clock genes in the skeletal muscle tissue. Daily oscillations in clock gene expression in skeletal muscle have been shown to correlate with daily variation in exercise performance.6 Moreover, metabolic pathways regulated by clock genes were identified within skeletal muscle, including those involved in glucose uptake, lipid metabolism and myokine secretion.7,8,9 A large-scale transcriptomic analysis revealed the rhythmic clock-driven pathways in human skeletal muscle that affect ~8% of all muscle genes.10 Furthermore, the skeletal muscle molecular clocks might account for the adaptations of training at a specific time of day,2,11,12,13 proposing a link between molecular clocks in skeletal muscle and exercise performance throughout the day.14 A well-synchronized circadian system is a manifestation of an individual's health. Aging is characterized by a gradual deterioration of the circadian timing function.15 The underlying mechanisms are not entirely understood, but the role of circadian molecular clocks at different levels and at disrupting the central network is assumed.16 Melatonin is an important sleep/wake cycle regulator in diurnal species, including humans.17,18 Ageing, causing melatonin synthesis decrease, adversely affects an individual's sleep.17 Melatonin is continuously metabolised to 6-sulfatoxymelatonin (aMT6s) and excreted in urine.17 Interestingly, an aging-related attenuation of circadian rhythms might be at least partially related to an inflammatory milieu of the aging organism. Indeed, cytokines can affect the expression of clock genes,19,20 and conversely, clock proteins can directly or indirectly affect the inflammatory response.21,22 Therefore, inflammation and disruption of circadian rhythms in the elderly seem to be mutually interconnected processes.21 Thus, regular physical activity has been considered an effective tool that may attenuate age-related inflammatory processes and eliminate their negative impact on various body systems. In addition, exercise at distinct times of the day can act as a so-called Zeitgeber and entrain the peripheral circadian clock.24 Moreover, the potential of regular exercise/physical activity as a possible preventive and even therapeutic tool for disease management through maintaining or improving circadian organisation during aging has been increasingly discussed.23,25 For instance, an entrainment of the renal molecular clock by exercise can impose beneficial effects on health in aged populations through improved circadian regulation of renal blood flow, fluid balance, and blood pressure.26 Regular physical activity improves circadian misalignment in both young and old mammals, although the precise mechanisms for this protection remain poorly described. On the other hand, exercise performed at certain time windows, especially at the beginning of the night and during the night may provide limited benefits. For example, nocturnal exercise induced greater gastrointestinal functional perturbations and symptoms concomitated by significant elevation in cortisol levels, compared with diurnal exercise.27 Clearly, there is a lack of direct evidence for definitive conclusions and physical activity is usually only an umbrella term for a set of different forms of exercise, which may differ in their impacts on the body. Till now, there have been no studies examining lifelong endurance exercise as a potential modality for improving the circadian timing function in aging, which can mediate positive effects on the overall health and well-being in the elderly.
The purpose of the present comparative cross-sectional study is to examine the benefits and risks of lifelong endurance exercise on age-dependent changes in the circadian time-keeping function, the performance of the muscular system and health status.
Materials and Methods
Study design
All procedures will be assessed within 3 visits, and the time overview of procedures is shown in Figure 1.
Trial status
At the time of the first submission of the protocol, the trial was in the phase of participants’ recruitment. The recruitment began in September 2022 and the last part of data collection is expected to end in April 2024.
Participants
The study will involve a total of 64 healthy male participants, recruited from the Bratislava region, Slovakia via fliers, social media and webpages. Participants will be divided into four groups:
group 1, young endurance-trained athletes (young athletes - YA; n=16, age range 20-30 years);
group 2, young, less active than recommended males (young sedentary - YS; n=16, age range 20-30 years),
group 3, master endurance-trained athletes (master athletes - MA; n=16, age range 65-75 years);
group 4, seniors less active than recommended (elderly sedentary - ES; n=16, age range 65-75 years, respectively.
For the YA and MA, the best 16 runners per group according to their 10km and/or marathon race time will be preferably selected.
Prior to study inclusion and signing the informed consent, the volunteers will be informed verbally, as well as in writing, about all procedures. Participants must meet the following inclusion criteria to participate in this study:
a) for athletic groups participants must: perform a running activity for more than 300 minutes per week; and have performed this activity for at least 3 years (young athletes) or at least 15 years (master athletes).
b) for sedentary groups (participants less active than recommended): no history of regular physical training and practice of no more than 150 minutes of moderate or 75 minutes of vigorous activity per week.
The standard inclusion criterion for every group will be body mass index (BMI index; range 18.5-30 kg/m2).
Exclusion criteria will be recent or current infection, physical disabilities, malignant disease, cardiovascular or metabolic or autoimmune diseases, malnutrition, and pharmacological interference (e.g., steroids, nonsteroidal anti-inflammatory agents, immunosuppressive and antineoplastic drugs).
Fig 1.
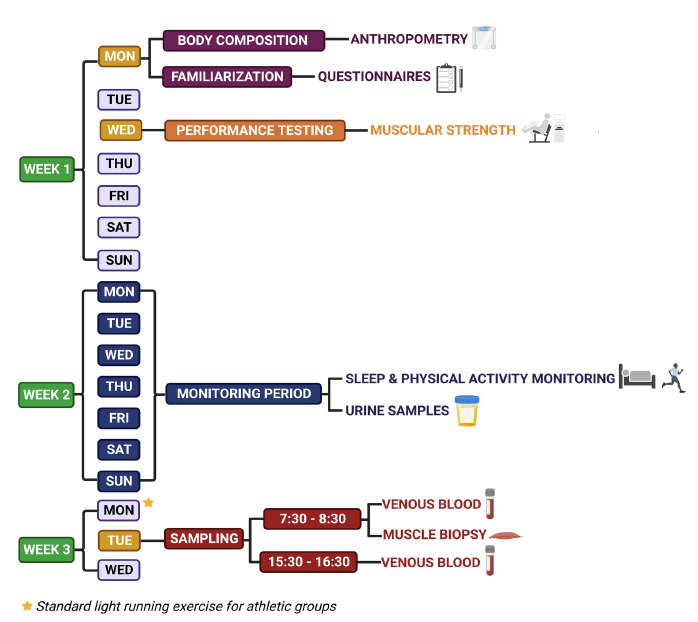
Study Design Timeline. Created with BioRender.com
This study has been approved by the Ethical Committee of the University Hospital Bratislava -Hospital of Ladislav Dérer, the Academician (number 31/2020) following the ethical standards of the Helsinki Declaration (2000) and its later amendments.28 The study is registered under the Clinical trials gov. registration number: NCT05053282. The participants will be fully informed about the nature and possible risks of all procedures before they are asked to provide written informed consent.
Familiarization
A familiarization session will be held 2 days before performance testing. Firstly, participants will bring a signed informed consent form and complete the questionnaires. Afterwards, each participant will undergo a medical check, consisting of measurements of heart rate, blood pressure, registration of resting ECG, body composition parameters, and a medical history to be taken by a physician. After the medical check, participants will be familiarized with each testing procedure and equipment.
The primary outcome
The primary outcome of the study is the quantification of 6-sulphatoxymelatonin (aMT6s) levels, a major urinary metabolite of melatonin, a hormone known to participate in the regulation of sleep cycles.18 Urine aMT6s exhibits robust circadian variation and can serve as a marker of the circadian phase.29
The urine samples will be collected during the second monitoring week (Figure 1). The participants will be instructed to collect urine samples in the evening from 18:00 until sleep time to monitor the evening rise of melatonin production and again immediately after waking up (the first and second voiding) to monitor a night-time melatonin surge. Each sample will be stored in the participant´s refrigerator and brought to the laboratory on the days when blood and biopsies will be collected. The concentrations of urine aMT6s will be measured using a melatonin sulfate ELISA kit (DRG Instruments, Marburg, Germany). The aMT6s levels will be normalized against creatinine, which will be determined by a quantitative enzymatic method for in vitro determination of creatinine in human serum, plasma, and urine (Erba Lachema, Brno, Czech Republic).
Secondary outcome measures
Sleep/activity rhythms and sleep quality
Sleep/activity rhythms and sleep quality, including sleep efficiency, will be measured by the MotionWatch8© wrist-worn actigraphy unit (MW8) (CamNtech; Cambridge, UK).30 Participants will be instructed to wear the MW8 on a non-dominant wrist during the entire 7-day period in the second monitoring week (Figure 1). They will be asked to press the event marker button on MW8 each evening when they lay in the bed and start trying to sleep, as well as in the morning when they finish sleeping. Moreover, participants will complete a Sleep Diary each morning to record and provide information about the times of their sleep onset/offset and also the level of sleepiness upon awakening.
Obtained data will be analysed to estimate circadian rhythm parameters, such as the amplitude (peak-to-nadir difference) and acrophase (time of peak activity).31 In addition, using the MotionWare software, different parameters of sleep quality, including sleep duration (total time spent sleeping), sleep latency (the time between lights-off and the sleep onset), sleep efficiency (the ratio of total sleep time to the time in bed), and fragmentation index (a measure of sleep interruptions) will be calculated.
Questionnaires
At the familiarization session, participants will be asked to complete the following questionnaires: 1) The Daily Activity Behaviour Questionnaire (DABQ), 2) The Aging Males Symptoms Scale (AMS), 3) The Munich ChronoType Questionnaire (MCTQ), and 4) Circadian Type Questionnaire.32 The questionnaires were translated to the Slovak language according to Sousa & Rojjanasrirat, 201133 and will be evaluated separately.
Body composition
Body height will be measured and determined to the nearest centimeter on a digital free-standing stadiometer. Body weight will be measured on a digital scale, with a participant being barefoot and wearing underwear (Body 230, InBody CO., Lt., Cerritos, USA).
Muscular strength
Maximal voluntary contraction (MVC – peak torque) and rate of torque development (RTD) of both isometric extension and flexion on the knee dynamometer (ARS dynamometry, S2P Ltd, Ljubljana, Slovenia) will be assessed.34 In all tests, participants will perform 2 warm-up isometric trials of duration 3 – 5 seconds with an intensity between 50% and 80% of maximal effort with a 30-second rest interval.35 Subsequently, three trials will be performed with maximal voluntary effort. The participants will be instructed to push/pull as fast and hard as possible and hold for 5 seconds against the lever arms.36,37 The rest interval between the trials will be 90 seconds. Monitored parameters will be MVC and RTD in four intervals of 0-50, 0-100, 0-150, and 0-200 milliseconds, respectively. The trials with the highest values will be saved for further analyses.
Blood and tissue collection and processing
The venous blood and muscle biopsies will be collected in the third week of the study, on Tuesday, 13 days after the physical performance testing session (Figure 1). The participants from the sedentary groups will be instructed to avoid any strenuous physical activity 48 hours before the procedures and participants from athletic groups will be asked to avoid standardized moderate-intensity running training 24 hours before the procedures. The procedures will be performed by a nurse and medical doctor at the hospital between 7:30 h and 8:00 h in the morning. Venous blood collection will be followed by the muscle biopsy procedure. Morning blood and biopsy samples will be taken after overnight fasting. Plain water consumption will be allowed before and during the sampling. Standard meals will be given at 10:00 h and 13:00 h. Eight hours after the morning of sampling, the participants will undergo the second blood collection between 15:30 h and 16:00 h.
Venous blood will be collected into K3EDTA and serum tubes. Two blood samples will be sent to a commercial medical laboratory to analyse blood count and serum biochemistry. Other blood samples will be processed in our laboratory. After centrifugation (2500g at 12°C for 15 minutes), plasma will be separated and stored at -80 °C for further analyses.
The muscle biopsy will be performed with the participant in a supine position and under local anesthesia (2% lidocaine).
A percutaneous muscle biopsy technique will be applied using the sterile Bergström needle (Bergström-Stille, Sweden, 5mm) with manual suction to obtain muscle specimens (approximately 80 mg) from the mid-section of the left m. vastus lateralis.35,38 Visible connective tissue and fat will be removed, and the muscle samples will be snap-frozen in liquid nitrogen and stored at -80°C until molecular analyses.
Biochemistry measures
Basic biochemical parameters, including serum glucose, urea, creatinine, uric acid, total proteins, albumins, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triacylglycerols will be analysed by a commercial medical laboratory.
Plasma hormone and immune measures
Plasma levels of pro- and anti-inflammatory markers (e.g., interleukin-1β, interleukin-4, interleukin-6, interleukin-10, tumor necrosis factor-α, C-reactive protein), as well as reproductive and metabolic hormones, which show a distinct circadian variation in the circulation (e.g., testosterone, steroid hormone binding globulin, cortisol, leptin, adiponectin and thyroid hormones), will be determined using commercial enzyme-linked immunosorbent assay kits (ELISA, Cloud-Clone Corp., USA), following the manufacturer's instructions.
Gene expression
Muscle tissue will be used to analyze gene expression of inflammatory markers, markers of muscle differentiation and atrophy, and molecular clock components. Total RNA will be isolated from the muscle tissue with TRI Reagent (Molecular Research Center, Cincinnati, OH, USA), according to the manufacturer's instructions. The quantity, purity, and integrity of the isolated RNA will be measured using a NanoDrop One spectrophotometer (Thermo Fisher Scientific) and a 2100 Bioanalyzer (Agilent Technologies, USA). For the synthesis of complementary DNA, a Maxima cDNA Synthesis Kit (Thermo Fisher Scientific, USA) will be used. Amplification of cDNA will be performed using Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific), and the CFX Connect real-time PCR detection system (Bio-Rad, USA). The relative expression of the target and reference genes will be calculated using a standard curve method. The expression of the target genes will be normalized to the geometric mean of at least two reference genes and to the expression of ribosomal protein lateral stalk subunit P0 (RPLP0), as done in previous studies examining human skeletal muscle.39
Statistical analyses
The descriptive parameters will be reported as mean values ± standard deviations (SD) and 95% of the confidence interval (95%CI). Data will be assessed for normal distribution and homogeneity of variances, using the Shapiro‐Wilk test, Levene's test, visual check of histograms, and Q‐Q plots.
A two-way ANOVA with Bonferroni post hoc correction will be used to determine differences between the groups in all measured parameters. Cohen's d effect size (ES) will be used to calculate between-group differences and will be interpreted using the following thresholds: 0-0.2 was trivial, 0.2-0.6 small, 0.6-1.2 moderate, 1.2-2.0 large, 2.0-4.0 very large, and > 4.0 extremely large effect.40
Association analyses will be performed using the Pearson product-moment correlation coefficient. The strength of the relationship will be determined using the following criteria: 0.1, small; 0.3, moderate; 0.5, large; 0.7, very large; 0.9, nearly perfect >0.9.41
Sample size
A sample size of 16 participants was predicted to provide a statistical power of 80% considering a type I error of 0.05.
Acknowledgments
None.
List of acronyms
- ANOVA
One-way analysis of variance
- aMT6s
6-sulphatoxymelatonin
- YA
Young athletes
- YS
Young sedentary
- MA
Master athletes
- ES
Elderly sedentary
- BMI
Body Mass Index
- ELISA
enzyme-linked immunosorbent assay kits
- MW8
Motion Watch 8
- MVC
Maximal voluntary contraction
- RTD
rate of torque development
- cDNA
copy DNA
Funding Statement
Funding: The study was supported by the cross-border cooperation program INTERREG V-A Slovakia – Austria through the project Centre of Active Ageing - Competence Centre for Health, Prevention and Active Ageing (acronym CAA, ITMS2014+ 305041X157) funded by European Regional Development Fund (partners: Faculty of Physical Education and Sports, Comenius University in Bratislava, Slovakia; Institute for Physical Medicine and Rehabilitation, Physiko- & Rheumatherapie GmbH, St. Pölten, Austria) and Grant no. APVV-210164 funded by Slovak Research and Development Agency.
Contributor Information
Genc Berisha, Email: genc.berisha@uniba.sk.
Michal Zeman, Email: michal.zeman@uniba.sk.
Dušan Hamar, Email: dusan.hamar@uniba.sk.
Ján Cvečka, Email: jan.cvecka@uniba.sk.
Veronika Tirpáková, Email: veronika.tirpakova@uniba.sk.
Matej Vajda, Email: matej.vajda@uniba.sk.
Ľudmila Oreská, Email: ludmila.oreska@uniba.sk.
Alena Černáčková, Email: alena.cernackova@uniba.sk.
Martin Čupka, Email: martin.cupka@uniba.sk.
Nejc Šarabon, Email: nejc.sarabon@fvz.upr.si.
Feliciano Protasi, Email: feliciano.protasi@unich.it.
Sandra Zampieri, Email: sanzamp@unipd.it.
Helmut Kern, Email: helmut@kern-reha.at.
Stefan Lofler, Email: stefan.Loefler@rehabilitation.lbg.ac.at.
Antonio Musaro, Email: antonio.Musaro@uniroma1.it.
Katarína Stebelová, Email: katarina.stebelova@uniba.sk.
Monika Okuliarová, Email: monika.okuliarova@uniba.sk.
References
- 1.Giacomoni M, Edwards B, Bambaeichi E. Gender differences in the circadian variations in muscle strength assessed with and without superimposed electrical twitches. Ergonomics. 2005. Sep 15-Nov 15;48(11-14):1473-87. doi: 10.1080/00140130500101452. PMID: 16338714. [DOI] [PubMed] [Google Scholar]
- 2.Küüsmaa M, Sedliak M, Häkkinen K. Effects of time-of-day on neuromuscular function in untrained men: Specific responses of high morning performers and high evening performers. Chronobiol Int. 2015;32(8):1115-24. doi: 10.3109/07420528.2015.1065269. Epub 2015 Sep 11. PMID: 26361893. [DOI] [PubMed] [Google Scholar]
- 3.Mora-Rodríguez R, García Pallarés J, López-Samanes Á, Ortega JF, Fernández-Elías VE. Caffeine ingestion reverses the circadian rhythm effects on neuromuscular performance in highly resistance-trained men. PLoS One. 2012;7(4):e33807. doi: 10.1371/journal.pone.0033807. Epub 2012 Apr 4. PMID: 22496767; PMCID: PMC3319538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedliak M, Finni T, Cheng S, Haikarainen T, Häkkinen K. Diurnal variation in maximal and submaximal strength, power and neural activation of leg extensors in men: multiple sampling across two consecutive days. Int J Sports Med. 2008. Mar;29(3):217-24. doi: 10.1055/s-2007-965125. Epub 2007 Jul 5. PMID: 17614012. [DOI] [PubMed] [Google Scholar]
- 5.Amaro-Gahete FJ, Jurado-Fasoli L, Triviño AR, Sanchez-Delgado G, De-la-O A, Helge JW, Ruiz JR. Diurnal Variation of Maximal Fat-Oxidation Rate in Trained Male Athletes. Int J Sports Physiol Perform. 2019. Sep 1;14(8):1140-1146. doi: 10.1123/ijspp.2018-0854. PMID: 30702364. [DOI] [PubMed] [Google Scholar]
- 6.Basti A, Yalçin M, Herms D, Hesse J, Aboumanify O, Li Y, Aretz Z, Garmshausen J, El-Athman R, Hastermann M, Blottner D, Relógio A. Diurnal variations in the expression of core-clock genes correlate with resting muscle properties and predict fluctuations in exercise performance across the day. BMJ Open Sport Exerc Med. 2021. Feb 10;7(1):e000876. doi: 10.1136/bmjsem-2020-000876. PMID: 33680499; PMCID: PMC7878143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyar KA, Ciciliot S, Wright LE, Biensø RS, Tagliazucchi GM, Patel VR, Forcato M, Paz MI, Gudiksen A, Solagna F, Albiero M, Moretti I, Eckel-Mahan KL, Baldi P, Sassone-Corsi P, Rizzuto R, Bicciato S, Pilegaard H, Blaauw B, Schiaffino S. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2013. Oct 23;3(1):29-41. doi: 10.1016/j.molmet.2013.10.005. Erratum in: Mol Metab. 2014. Dec;3(9): 857. PMID: 24567902; PMCID: PMC3929910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrin L, Loizides-Mangold U, Skarupelova S, Pulimeno P, Chanon S, Robert M, Bouzakri K, Modoux C, Roux-Lombard P, Vidal H, Lefai E, Dibner C. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab. 2015. Aug 6;4(11):834-45. doi: 10.1016/j.molmet.2015.07.009. PMID: 26629407; PMCID: PMC4632112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Moorsel D, Hansen J, Havekes B, Scheer FAJL, Jörgensen JA, Hoeks J, Schrauwen-Hinderling VB, Duez H, Lefebvre P, Schaper NC, Hesselink MKC, Staels B, Schrauwen P. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab. 2016. Jul 1;5(8):635-645. doi: 10.1016/j.molmet.2016.06.012. PMID: 27656401; PMCID: PMC5021670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrin L, Loizides-Mangold U, Chanon S, Gobet C, Hulo N, Isenegger L, Weger BD, Migliavacca E, Charpagne A, Betts JA, Walhin JP, Templeman I, Stokes K, Thompson D, Tsintzas K, Robert M, Howald C, Riezman H, Feige JN, Karagounis LG, Johnston JD, Dermitzakis ET, Gachon F, Lefai E, Dibner C. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elife. 2018. Apr 16;7:e34114. doi: 10.7554/eLife.34114. PMID: 29658882; PMCID: PMC5902165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedliak M, Finni T, Cheng S, Kraemer WJ, Häkkinen K. Effect of time-of-day-specific strength training on serum hormone concentrations and isometric strength in men. Chronobiol Int. 2007;24(6):1159-77. doi: 10.1080/07420520701800686. PMID: 18075805. [DOI] [PubMed] [Google Scholar]
- 12.Sedliak M, Finni T, Cheng S, Lind M, Häkkinen K. Effect of time-of-day-specific strength training on muscular hypertrophy in men. J Strength Cond Res. 2009. Dec;23(9):2451-7. doi: 10.1519/JSC.0b013e3181bb7388. PMID: 19910830. [DOI] [PubMed] [Google Scholar]
- 13.Sedliak M, Zeman M, Buzgó G, Cvecka J, Hamar D, Laczo E, Okuliarova M, Vanderka M, Kampmiller T, Häkkinen K, Ahtiainen JP, Hulmi JJ, Nilsen TS, Wiig H, Raastad T. Morphological, molecular and hormonal adaptations to early morning versus afternoon resistance training. Chronobiol Int. 2018. Apr;35(4):450-464. doi: 10.1080/07420528.2017.1411360. Epub 2017 Dec 28. PMID: 29283292. [DOI] [PubMed] [Google Scholar]
- 14.Mirizio GG, Nunes RSM, Vargas DA, Foster C, Vieira E. Time-of-Day Effects on Short-Duration Maximal Exercise Performance. Sci Rep. 2020. Jun 11;10(1):9485. doi: 10.1038/s41598-020-66342-w. PMID: 32528038; PMCID: PMC7289891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelissen G, Otsuka K. Chronobiology of Aging: A Mini-Review. Gerontology. 2017;63(2):118-128. doi: 10.1159/000450945. Epub 2016 Oct 22. PMID: 27771728. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Warman GR, Cheeseman JF. The functional changes of the circadian system organization in aging. Ageing Res Rev. 2019. Jul;52:64-71. doi: 10.1016/j.arr.2019.04.006. Epub 2019 Apr 29. PMID: 31048031. [DOI] [PubMed] [Google Scholar]
- 17.Cipolla-Neto J, Amaral FGD. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr Rev. 2018. Dec 1;39(6):990-1028. doi: 10.1210/er.2018-00084. PMID: 30215696. [DOI] [PubMed] [Google Scholar]
- 18.Khullar A. The role of melatonin in the circadian rhythm sleep-wake cycle. Psychiatric Times. 2012;29(7):26-27,30-32. [Google Scholar]
- 19.Yoshida K, Hashiramoto A, Okano T, Yamane T, Shibanuma N, Shiozawa S. TNF-α modulates expression of the circadian clock gene Per2 in rheumatoid synovial cells. Scand J Rheumatol. 2013;42(4):276-80. doi: 10.3109/03009742.2013.765031. Epub 2013 Mar 16. PMID: 23496259. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Nakai A, Kaneshiro K, Hashimoto N, Suzuki K, Uchida K, Hashimoto T, Kawasaki Y, Tateishi K, Nakagawa N, Shibanuma N, Sakai Y, Hashiramoto A. TNF-α induces expression of the circadian clock gene Bmal1 via dual calcium-dependent pathways in rheumatoid synovial cells. Biochem Biophys Res Commun. 2018. Jan 8;495(2):1675-1680. doi: 10.1016/j.bbrc.2017.12.015. Epub 2017 Dec 5. PMID: 29217191. [DOI] [PubMed] [Google Scholar]
- 21.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012. Jul 31;109(31):12662-7. doi: 10.1073/pnas.1209965109. Epub 2012 Jul 9. PMID: 22778400; PMCID: PMC3411996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, Antoch MP. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc Natl Acad Sci U S A. 2012. Sep 11;109(37):E2457-65. doi: 10.1073/pnas.1206274109. Epub 2012 Aug 15. PMID: 22895791; PMCID: PMC3443185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Souza Teixeira AA, Lira FS, Rosa-Neto JC. Aging with rhythmicity. Is it possible? Physical exercise as a pacemaker. Life Sci. 2020. Nov 15;261:118453. doi: 10.1016/j.lfs.2020.118453. Epub 2020 Sep 18. PMID: 32956663; PMCID: PMC7500276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabriel BM, Zierath JR. Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol. 2019. Apr;15(4):197-206. doi: 10.1038/s41574-018-0150-x. PMID: 30655625. [DOI] [PubMed] [Google Scholar]
- 25.Choi Y, Cho J, No MH, Heo JW, Cho EJ, Chang E, Park DH, Kang JH, Kwak HB. Re-Setting the Circadian Clock Using Exercise against Sarcopenia. Int J Mol Sci. 2020. Apr 28;21(9):3106. doi: 10.3390/ijms21093106. PMID: 32354038; PMCID: PMC7247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt EE, Johnson EC, Yusifova M, Bruns DR. The renal molecular clock: broken by aging and restored by exercise. Am J Physiol Renal Physiol. 2019. Nov 1;317(5):F1087-F1093. doi: 10.1152/ajprenal.00301.2019. Epub 2019 Aug 28. PMID: 31461350; PMCID: PMC6879930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaskell SK, Rauch CE, Parr A, Costa RJS. Diurnal versus Nocturnal Exercise-Effect on the Gastrointestinal Tract. Med Sci Sports Exerc. 2021. May 1;53(5):1056-1067. doi: 10.1249/MSS.00000 00000002546. PMID: 33065594. [DOI] [PubMed] [Google Scholar]
- 28.Youngstedt SD, Elliott JA, Kripke DF. Human circadian phase-response curves for exercise. J Physiol. 2019. Apr;597(8):2253-2268. doi: 10.1113/JP276943. Epub 2019 Mar 18. PMID: 30784068; PMCID: PMC6462487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbaz M, Yauy K, Metlaine A, Martoni M, Léger D. Validation of a new actigraph motion watch versus polysomnography on 70 healthy and suspected sleepdisordered subjects. In Universite Paris Descartes. 2012. (Vol. 21, Issue 2001). [Google Scholar]
- 30.Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, Wise M, Johnson SF; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003. May 1;26(3):337-41. doi: 10.1093/sleep/26.3.337. PMID: 12749556. [DOI] [PubMed] [Google Scholar]
- 31.Folkard S, Monk TH, Lobban MC. Towards a predictive test of adjustment to shift work. Ergonomics. 1979. Jan;22(1):79-91. doi: 10.1080/00140137908924591. PMID: 436816. [DOI] [PubMed] [Google Scholar]
- 32.Sousa VD, Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract. 2011. Apr;17(2):268-74. doi: 10.1111/j.1365-2753.2010.01434.x. Epub 2010 Sep 28. PMID: 20874835. [DOI] [PubMed] [Google Scholar]
- 33.Bily W, Franz C, Trimmel L, Loefler S, Cvecka J, Zampieri S, Kasche W, Sarabon N, Zenz P, Kern H. Effects of Leg-Press Training With Moderate Vibration on Muscle Strength, Pain, and Function After Total Knee Arthroplasty: A Randomized Controlled Trial. Arch Phys Med Rehabil. 2016. Jun;97(6):857-65. doi: 10.1016/j.apmr.2015.12.015. Epub 2016 Jan 4. PMID: 26763947. [DOI] [PubMed] [Google Scholar]
- 34.Kralik M, Cvecka J, Buzgo G, Putala M, Ukropcova B, Ukropec J, Killinger Z, Payer J, Kollarik B, Bujdak P, Raastad T, Sedliak M. Strength training as a supplemental therapy for androgen deficiency of the aging male (ADAM): study protocol for a three-arm clinical trial. BMJ Open. 2019. Sep 5;9(9):e025991. doi: 10.1136/bmjopen-2018-025991. PMID: 31492775; PMCID: PMC6731925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarabon N, Rosker J, Fruhmann H, Burggraf S, Loefler S, Kern H. Reliability of maximal voluntary contraction related parameters measured by a novel portable isometric knee dynamometer. Phys Medizin Rehabil Kurortmedizin. 2013;23(1). [Google Scholar]
- 36.Sarabon N, Loefler S, Cvecka J, Sedliak M, Kern H. Strength training in elderly people improves static balance: a randomized controlled trial. Eur J Transl Myol. 2013;23(3). [Google Scholar]
- 37.Shanely RA, Zwetsloot KA, Triplett NT, Meaney MP, Farris GE, Nieman DC. Human skeletal muscle biopsy procedures using the modified Bergström technique. J Vis Exp. 2014. Sep 10;(91):51812. doi: 10.3791/51812. PMID: 25285722; PMCID: PMC4828068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985). 2020. Jan 1;128(1):87-99. doi: 10.1152/japplphysiol.00495.2019. Epub 2019 Nov 21. PMID: 31751180; PMCID: PMC6985808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopkins WG. A scale of magnitudes for effect statistics. Sportscience. 2002;5. [Google Scholar]
- 40.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009. Jan;41(1):3-13. doi: 10.1249/MSS.0b013e31818cb278. PMID: 19092709. [DOI] [PubMed] [Google Scholar]
- 41.Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hróbjartsson A, Schulz KF, Parulekar WR, Krleza-Jeric K, Laupacis A, Moher D. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013. Jan 8;346:e7586. doi: 10.1136/bmj.e7586. PMID: 23303884; PMCID: PMC3541470. [DOI] [PMC free article] [PubMed] [Google Scholar]


