Abstract
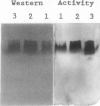
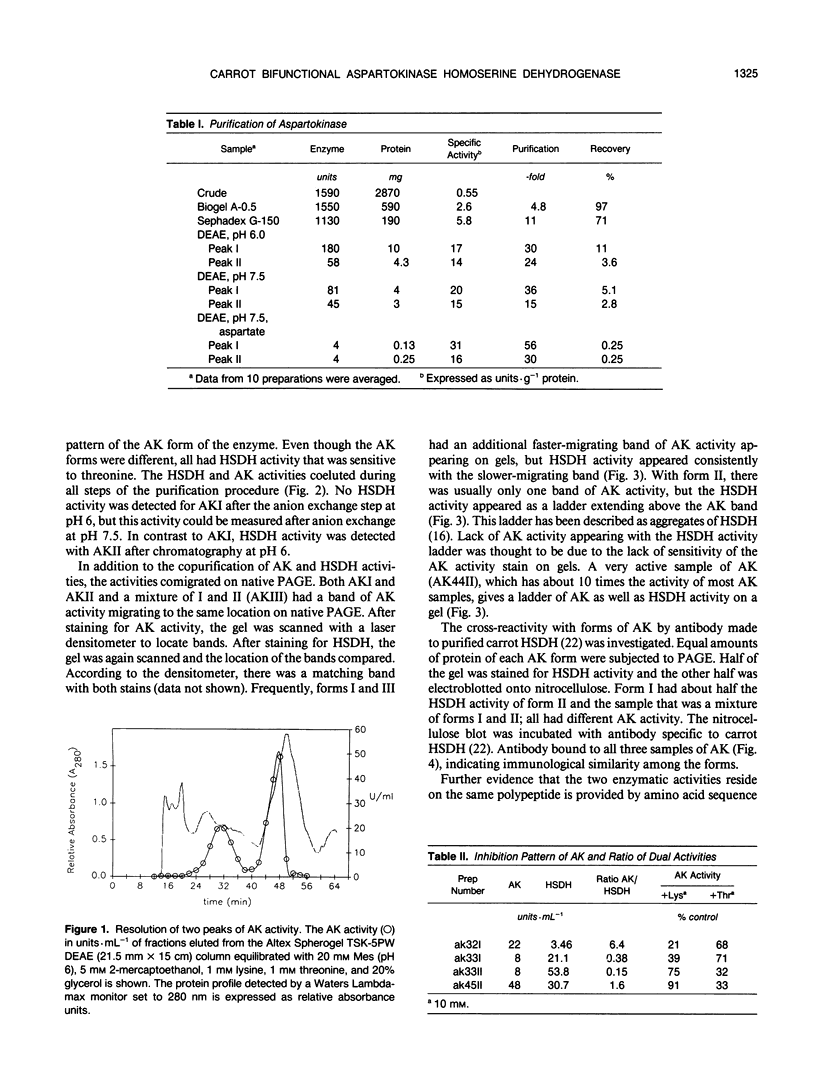
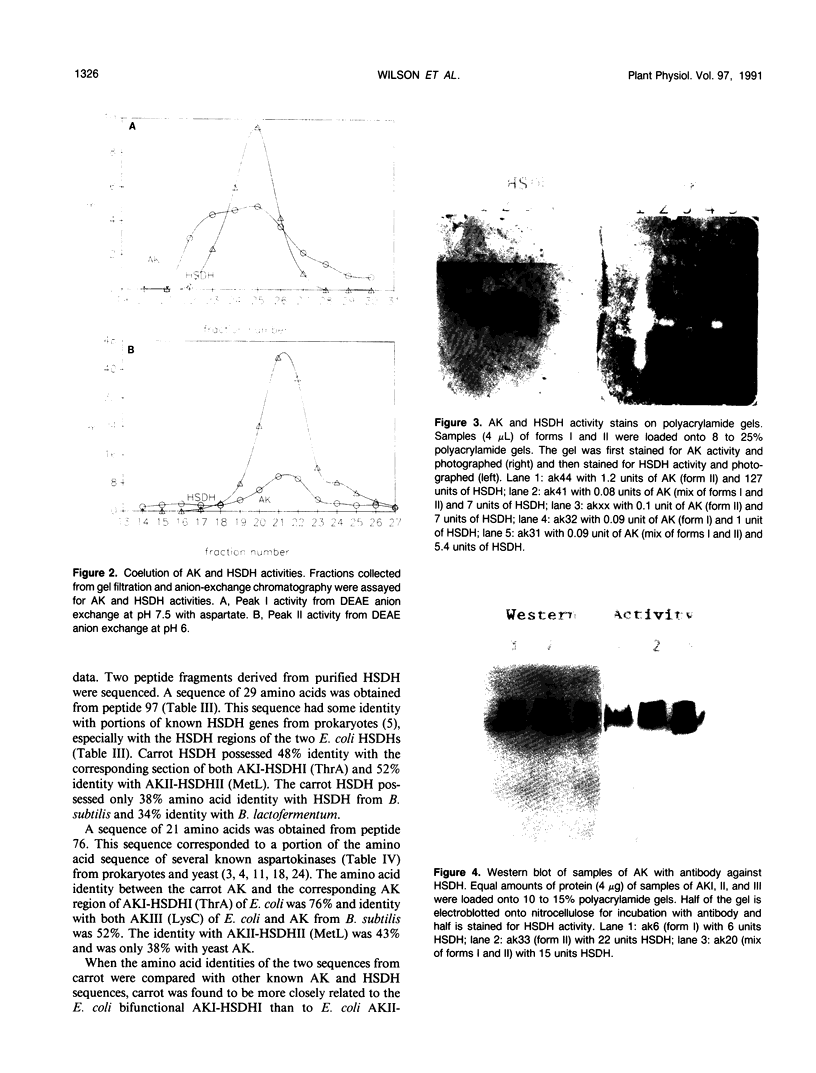
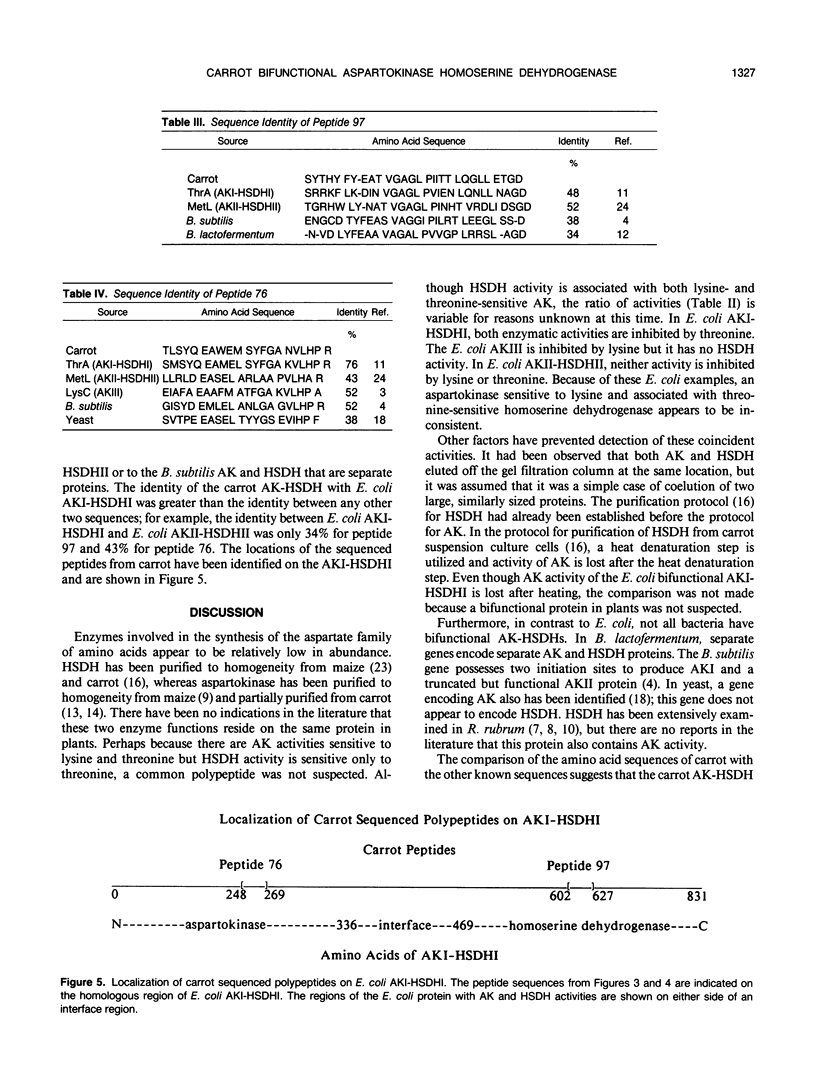
We have purified homoserine dehydrogenase to homogeneity and subjected polypeptide fragments derived from digests of the protein to amino acid sequencing. The amino acid sequence of homoserine dehydrogenase from carrot (Daucus carota) indicates that in carrot both aspartokinase and homoserine dehydrogenase activities reside on the same protein. Additional evidence that aspartokinase and homoserine dehydrogenase reside on a bifunctional protein is provided by coelution of activities during purification steps and by enzyme-specific gel staining techniques. Highly purified fractions containing aspartokinase activity were stained for aspartokinase activity, homoserine dehydrogenase activity, and protein. These gels confirmed that aspartokinase activity and homoserine dehydrogenase activity were present on the same protein. This arrangement of aspartokinase and homoserine dehydrogenase activities residing on the same protein is also found in Escherichia coli, which has two bifunctional enzymes, aspartokinase I-homoserine dehydrogenase I and aspartokinase II-homoserine dehydrogenase II. The amino acid sequence of the major form of homoserine dehydrogenase from carrot cell suspension cultures most closely resembles that of the E. coli ThrA gene product aspartokinase I-homoserine dehydrogenase I.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondaryk R. P., Paulus H. Cloning and structure of the gene for the subunits of aspartokinase II from Bacillus subtilis. J Biol Chem. 1985 Jan 10;260(1):585–591. [PubMed] [Google Scholar]
- Bryan P. A., Cawley R. D., Brunner C. E., Bryan J. K. Isolation and characterization of a lysine-sensitive aspartokinase from a multicellular plant. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1211–1217. doi: 10.1016/0006-291x(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Cassan M., Parsot C., Cohen G. N., Patte J. C. Nucleotide sequence of lysC gene encoding the lysine-sensitive aspartokinase III of Escherichia coli K12. Evolutionary pathway leading to three isofunctional enzymes. J Biol Chem. 1986 Jan 25;261(3):1052–1057. [PubMed] [Google Scholar]
- Chen N. Y., Hu F. M., Paulus H. Nucleotide sequence of the overlapping genes for the subunits of Bacillus subtilis aspartokinase II and their control regions. J Biol Chem. 1987 Jun 25;262(18):8787–8798. [PubMed] [Google Scholar]
- DATTA P., GEST H. HOMOSERINE DEHYDROGENASE OF RHODOSPIRILLUM RUBRUM. PURIFICATION, PROPERTIES, AND FEEDBACK CONTROL OF ACTIVITY. J Biol Chem. 1965 Jul;240:3023–3033. [PubMed] [Google Scholar]
- Datta P. Homoserine dehydrogenase of Rhodospirillum rubrum. II. Purification and characterization of the enzyme. J Biol Chem. 1970 Nov 10;245(21):5779–5787. [PubMed] [Google Scholar]
- Davies H. M., Miflin B. J. Regulatory isoenzymes of aspartate kinase and the control of lysine and threonine biosynthesis in carrot cell suspension culture. Plant Physiol. 1978 Oct;62(4):536–541. doi: 10.1104/pp.62.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson S. B., Somers D. A., Gengenbach B. G. Purification and characterization of lysine-sensitive aspartate kinase from maize cell cultures. Plant Physiol. 1989 Dec;91(4):1602–1608. doi: 10.1104/pp.91.4.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. C., Datta P. Homoserine dehydrogenase of Rhodospirillum rubrum. Physical and chemical characterization. Eur J Biochem. 1978 Jan 16;82(2):453–461. doi: 10.1111/j.1432-1033.1978.tb12039.x. [DOI] [PubMed] [Google Scholar]
- Katinka M., Cossart P., Sibilli L., Saint-Girons I., Chalvignac M. A., Le Bras G., Cohen G. N., Yaniv M. Nucleotide sequence of the thrA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5730–5733. doi: 10.1073/pnas.77.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos L. M., del Real G., Aguilar A., Martín J. F. Nucleotide sequence of the homoserine dehydrogenase (thr A) gene of Brevibacterium lactofermentum. Nucleic Acids Res. 1987 Dec 23;15(24):10598–10598. doi: 10.1093/nar/15.24.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. F., Farrar M. J., Gray A. C. Purification and Interconversion of Homoserine Dehydrogenase from Daucus carota Cell Suspension Cultures. Plant Physiol. 1989 Dec;91(4):1569–1574. doi: 10.1104/pp.91.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. F., Widholm J. M. Expression of aspartokinase, dihydrodipicolinic acid synthase and homoserine dehydrogenase during growth of carrot cell suspension cultures on lysine- and threonine-supplemented media. Z Naturforsch C. 1979 Dec;34(12):1177–1185. doi: 10.1515/znc-1979-1216. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Nimmo G. A. A general method for the localization of enzymes that produce phosphate, pyrophosphate, or CO2 after polyacrylamide gel electrophoresis. Anal Biochem. 1982 Mar 15;121(1):17–22. doi: 10.1016/0003-2697(82)90551-6. [DOI] [PubMed] [Google Scholar]
- Rafalski J. A., Falco S. C. Structure of the yeast HOM3 gene which encodes aspartokinase. J Biol Chem. 1988 Feb 15;263(5):2146–2151. [PubMed] [Google Scholar]
- Sakano K. Derepression and Repression of Lysine-sensitive Aspartokinase during in Vitro Culture of Carrot Root Tissue. Plant Physiol. 1979 Mar;63(3):583–585. doi: 10.1104/pp.63.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano K., Komamine A. Change in the proportion of two aspartokinases in carrot root tissue in response to in vitro culture. Plant Physiol. 1978 Jan;61(1):115–118. doi: 10.1104/pp.61.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano F. J., Jordan R. L., Matthews B. F. Immunological characterization of in vitro forms of homoserine dehydrogenase from carrot suspension cultures. Plant Physiol. 1990 Feb;92(2):395–400. doi: 10.1104/pp.92.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter T. J., Connelly J. A., Gengenbach B. G., Wold F. Isolation and characterization of two homoserine dehydrogenases from maize suspension cultures. J Biol Chem. 1979 Feb 25;254(4):1349–1355. [PubMed] [Google Scholar]
- Zakin M. M., Duchange N., Ferrara P., Cohen G. N. Nucleotide sequence of the metL gene of Escherichia coli. Its product, the bifunctional aspartokinase ii-homoserine dehydrogenase II, and the bifunctional product of the thrA gene, aspartokinase I-homoserine dehydrogenase I, derive from a common ancestor. J Biol Chem. 1983 Mar 10;258(5):3028–3031. [PubMed] [Google Scholar]