Abstract
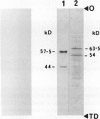
Leucoplast pyruvate kinase (PKp; EC 2.7.1.40) from endosperm of developing castor oil seeds (Ricinus communis L. cv Baker 296) appears to be highly susceptible to limited degradation by a cysteine endopeptidase during the purification of the enzyme or incubation of clarified homogenates at 4°C. Purified castor seed PKp was previously reported to consist of immunologically related 57.5 and 44 kilodalton subunits (Plaxton WC, Dennis DT, Knowles VL [1990] Plant Physiol 94: 1528-1534). By contrast, immunoreactive polypeptides of about 63.5 and 54 kilodaltons were observed when a western blot of an extract prepared under denaturing conditions was probed with affinity purified rabbit anti-(castor seed PKp) immunoglobulin G. Proteolytic activity against PKp was estimated by the disappearance of the 63.5 and 54 kilodalton subunits and the concomitant appearance of lower molecular mass immunoreactive degradation products during the incubation of clarified homogenates at 4°C. The presence of 2 millimolar dithiothreitol accelerated the degradation of PKp. The conservation of the 63.5 and 54 kilodalton subunits was observed after extraction of the enzyme in the presence of 1 millimolar p-hydroxymecuribenzoate, or 1 millimolar Nα-p-tosyl-l-lysine chloromethyl ketone, or 10 millimolar iodoacetate. These results reveal that a cysteine endopeptidase was responsible for the in vitro proteolysis of PKp. This endopeptidase is present throughout all stages of endosperm development. Its PKp-degrading activity, however, appears to be most pronounced in preparations from older endosperm. When lysates of purified leucoplasts were incubated at 4°C for up to 21 hours, no degradation of PKp was observed; this indicated an extra-leucoplastic localization for the cysteine endopeptidase. Although the in vivo subunit structure of PKp remains uniform throughout all stages of endosperm development, the large decrease in PK activity that accompanies castor seed maturation coincides with a marked reduction in the concentration of PKp.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baysdorfer C., Bassham J. A. Spinach pyruvate kinase isoforms : partial purification and regulatory properties. Plant Physiol. 1984 Feb;74(2):374–379. doi: 10.1104/pp.74.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley S. D., Plaxton W. C., Dennis D. T. Cloning and characterization of a cDNA for the cytosolic isozyme of plant pyruvate kinase: the relationship between the plant and non-plant enzyme. Plant Mol Biol. 1990 Oct;15(4):665–669. doi: 10.1007/BF00017842. [DOI] [PubMed] [Google Scholar]
- Blakeley S. D., Plaxton W. C., Dennis D. T. Relationship between the Subunits of Leucoplast Pyruvate Kinase from Ricinus communis and a Comparison with the Enzyme from Other Sources. Plant Physiol. 1991 Aug;96(4):1283–1288. doi: 10.1104/pp.96.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S. A., Hemmingsen S. M., Dennis D. T. Uptake and processing of the precursor to the small subunit of ribulose 1,5-bisphosphate carboxylase by leucoplasts from the endosperm of developing castor oil seeds. Plant Physiol. 1986 Jul;81(3):817–822. doi: 10.1104/pp.81.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ireland R. J., De Luca V., Dennis D. T. Characterization and kinetics of isoenzymes of pyruvate kinase from developing castor bean endosperm. Plant Physiol. 1980 Jun;65(6):1188–1193. doi: 10.1104/pp.65.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Bruno P. L. NADP-malate dehydrogenase from leaves of Zea mays: purification and physical, chemical, and kinetic properties. Arch Biochem Biophys. 1988 Feb 1;260(2):674–695. doi: 10.1016/0003-9861(88)90497-3. [DOI] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. I. Purification and physical and immunological characterization. Arch Biochem Biophys. 1989 Feb 15;269(1):219–227. doi: 10.1016/0003-9861(89)90103-3. [DOI] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. II. Kinetic and regulatory properties. Arch Biochem Biophys. 1989 Feb 15;269(1):228–238. doi: 10.1016/0003-9861(89)90104-5. [DOI] [PubMed] [Google Scholar]
- Miyadai K., Mae T., Makino A., Ojima K. Characteristics of ribulose-1,5-bisphosphate carboxylase/oxygenase degradation by lysates of mechanically isolated chloroplasts from wheat leaves. Plant Physiol. 1990 Apr;92(4):1215–1219. doi: 10.1104/pp.92.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C., Dennis D. T., Knowles V. L. Purification of leucoplast pyruvate kinase from developing castor bean endosperm. Plant Physiol. 1990 Dec;94(4):1528–1534. doi: 10.1104/pp.94.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C. Molecular and immunological characterization of plastid and cytosolic pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem. 1989 May 1;181(2):443–451. doi: 10.1111/j.1432-1033.1989.tb14745.x. [DOI] [PubMed] [Google Scholar]
- Plaxton W. C., Preiss J. Purification and Properties of Nonproteolytic Degraded ADPglucose Pyrophosphorylase from Maize Endosperm. Plant Physiol. 1987 Jan;83(1):105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C. Purification of pyruvate kinase from germinating castor bean endosperm. Plant Physiol. 1988 Apr;86(4):1064–1069. doi: 10.1104/pp.86.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podestá F. E., Plaxton W. C. Kinetic and regulatory properties of cytosolic pyruvate kinase from germinating castor oil seeds. Biochem J. 1991 Oct 15;279(Pt 2):495–501. doi: 10.1042/bj2790495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M., Tsujita M., Tomizawa H., Sakihama N., Kamei K., Oshino R. Proteolytic degradation of ferredoxin-NADP reductase during purification from spinach. Arch Biochem Biophys. 1990 May 15;279(1):97–103. doi: 10.1016/0003-9861(90)90467-d. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Proteases and Peptidases of Castor Bean Endosperm: Enzyme Characterization and Changes during Germination. Plant Physiol. 1978 Nov;62(5):746–750. doi: 10.1104/pp.62.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S., Wang M. Y. Extraction of proteins for sodium dodecyl sulfate-polyacrylamide gel electrophoresis from protease-rich plant tissues. Anal Biochem. 1984 May 15;139(1):100–103. doi: 10.1016/0003-2697(84)90394-4. [DOI] [PubMed] [Google Scholar]