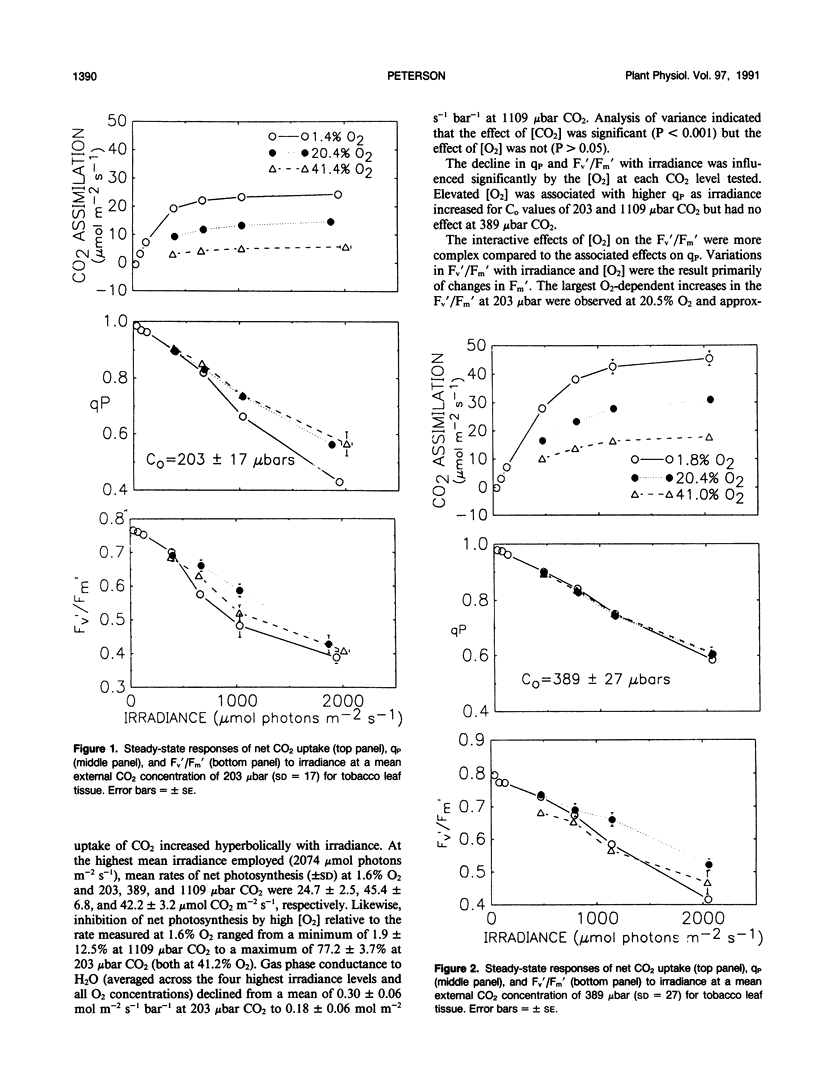
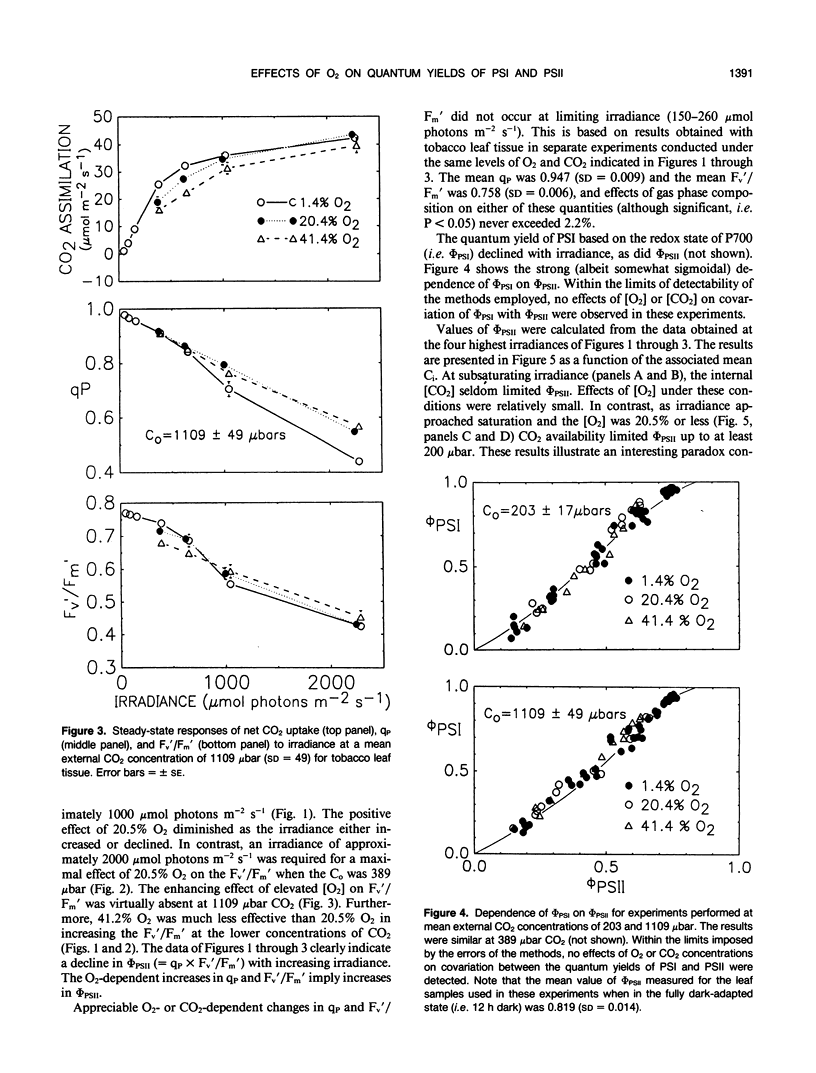
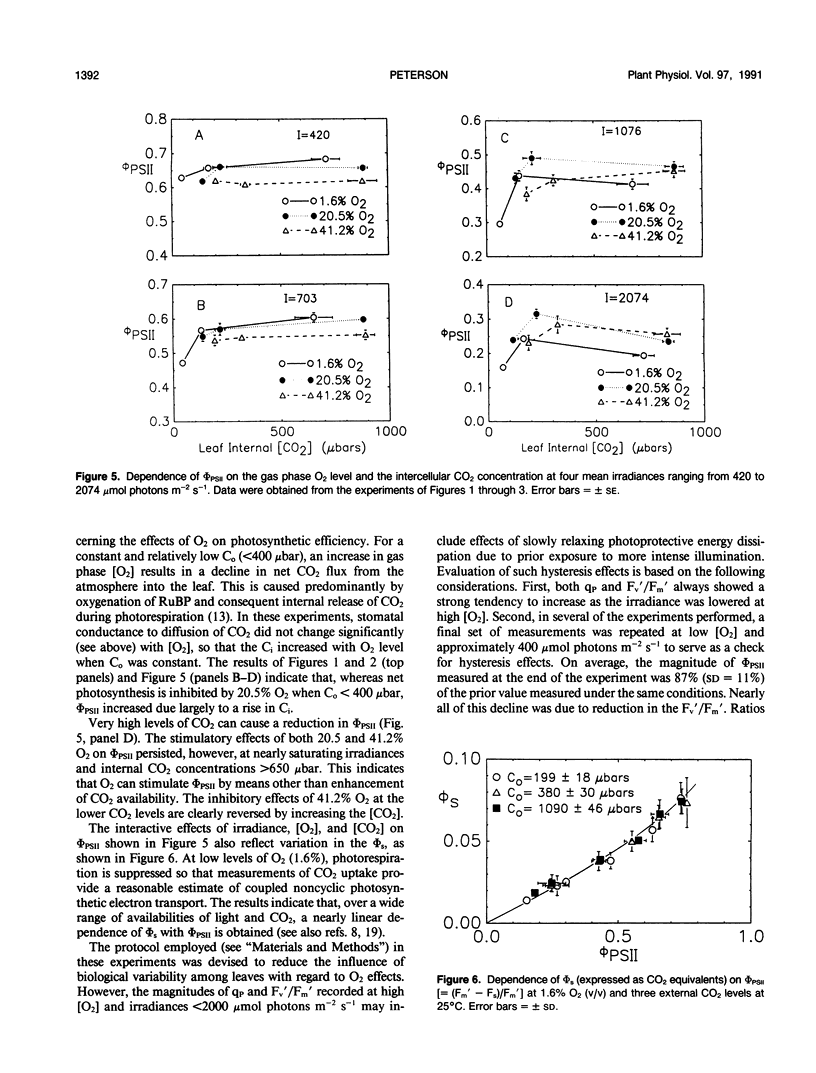
Abstract
The interactive effects of irradiance and O2 and CO2 levels on the quantum yields of photosystems I and II have been studied under steady-state conditions at 25°C in leaf tissue of tobacco (Nicotiana tabacum). Assessment of radiant energy utilization in photosystem II was based on changes in chlorophyll fluorescence yield excited by a weak measuring beam of modulated red light. Independent estimates of photosystem I quantum yield were based on the light-dark in vivo absorbance change at 830 nanometers, the absorption band of P700+. Normal (i.e. 20.5%, v/v) levels of O2 generally enhanced photosystem II quantum yield relative to that measured under 1.6% O2 as the irradiance approached saturation. Photorespiration is suspected to mediate such positive effects of O2 through increases in the availability of CO2 and recycling of orthophosphate. Conversely, at low intercellular CO2 concentrations, 41.2% O2 was associated with lower photosystem II quantum yield compared with that observed at 20.5% O2. Inhibitory effects of 41.2% O2 may occur in response to negative feedback on photosystem II arising from a build-up in the thylakoid proton gradient during electron transport to O2. Covariation between quantum yields of photosystems I and II was not affected by concentrations of either O2 or CO2. The dependence of quantum yield of electron transport to CO2 measured by gas exchange upon photosystem II quantum yield as determined by fluorescence was unaffected by CO2 concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., Chain R. K. Regulation of ferredoxin-catalyzed photosynthetic phosphorylations. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4961–4965. doi: 10.1073/pnas.72.12.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. Chlorophyll fluorescence as a probe for the determination of the photo-induced proton gradient in isolated chloroplasts. Biochim Biophys Acta. 1980 Jun 10;591(1):198–202. doi: 10.1016/0005-2728(80)90233-9. [DOI] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. C., Cheesbrough J. K., Walker D. A. Measurement of CO(2) and H(2)O Vapor Exchange in Spinach Leaf Discs : Effects of Orthophosphate. Plant Physiol. 1983 Jan;71(1):102–107. doi: 10.1104/pp.71.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975 Jan 31;376(1):105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Monteith J. L. Evaporation and environment. Symp Soc Exp Biol. 1965;19:205–234. [PubMed] [Google Scholar]
- Peterson R. B. Analysis of changes in minimal and maximal fluorescence yields with irradiance and o(2) level in tobacco leaf tissue. Plant Physiol. 1991 May;96(1):172–177. doi: 10.1104/pp.96.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Effects of Irradiance on the in Vivo CO(2):O(2) Specificity Factor in Tobacco Using Simultaneous Gas Exchange and Fluorescence Techniques. Plant Physiol. 1990 Nov;94(3):892–898. doi: 10.1104/pp.94.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Effects of water vapor pressure deficit on photochemical and fluorescence yields in tobacco leaf tissue. Plant Physiol. 1990 Mar;92(3):608–614. doi: 10.1104/pp.92.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D. O(2)-insensitive photosynthesis in c(3) plants : its occurrence and a possible explanation. Plant Physiol. 1985 May;78(1):71–75. doi: 10.1104/pp.78.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]


