Abstract
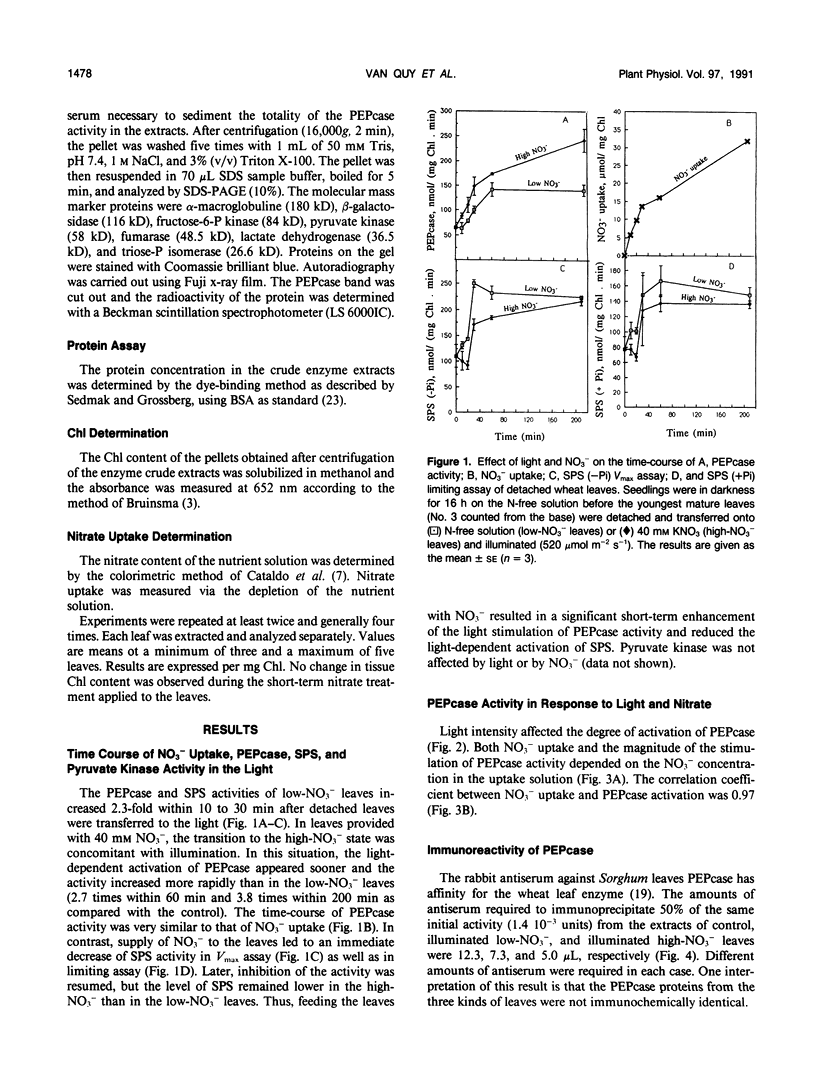
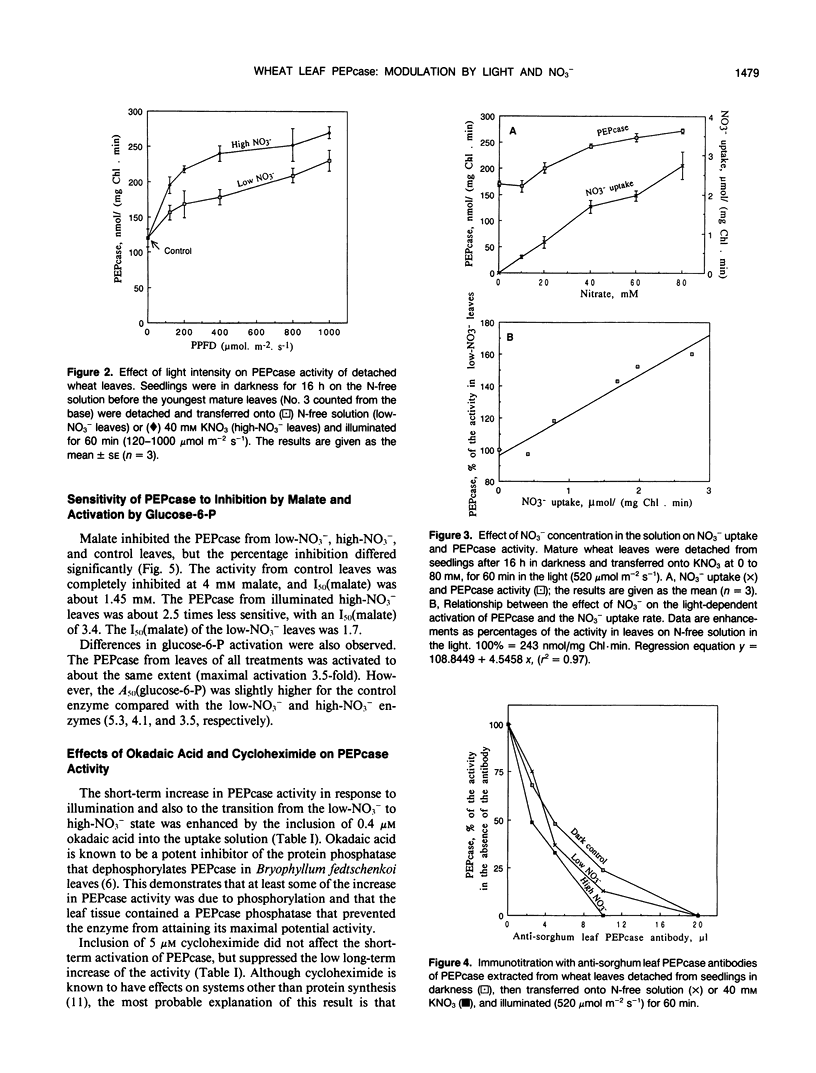
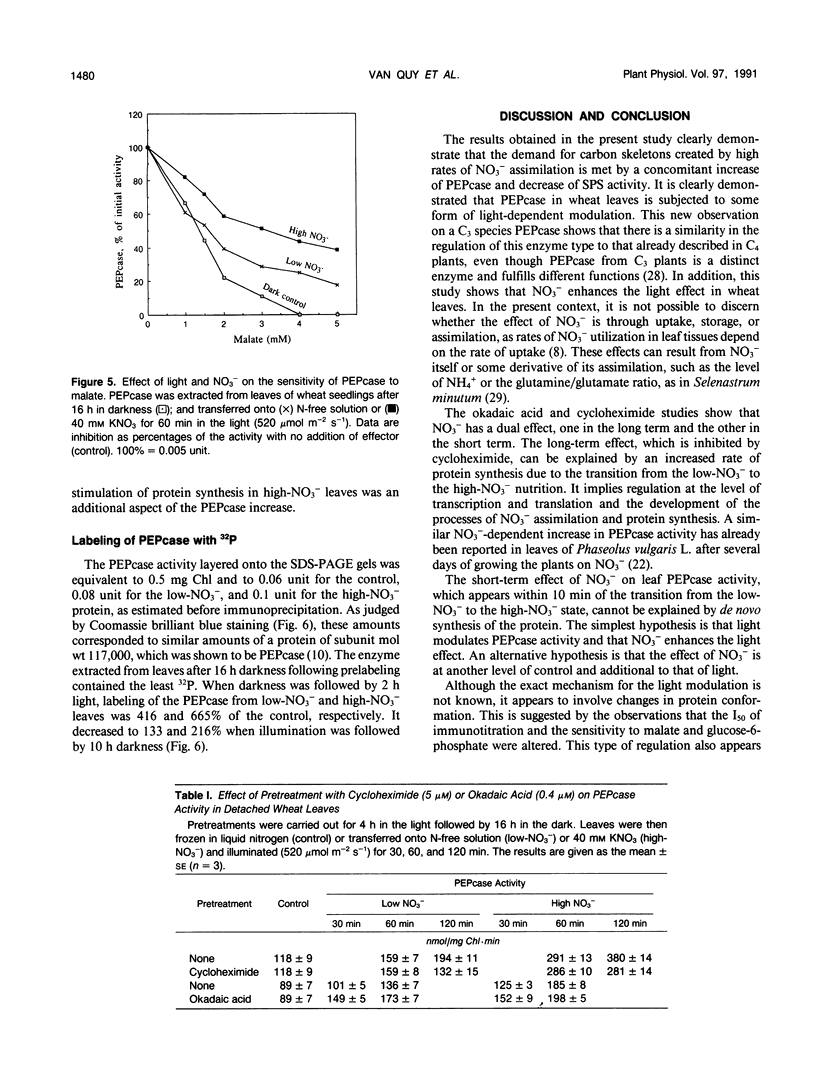
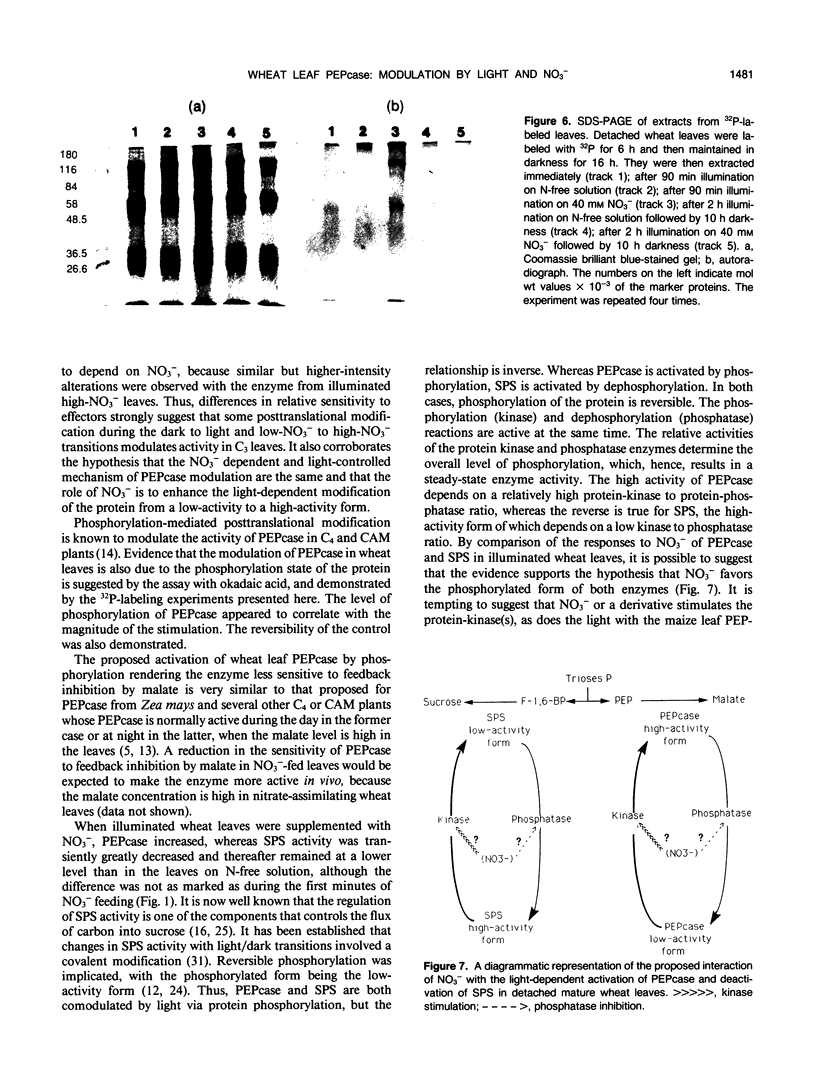
Phosphoenolpyruvate carboxylase (PEPcase) activity was studied in excised leaves of wheat (Triticum aestivum L.) in the dark and in the light, in presence of either N-free (low-NO3− leaves) or 40 millimolar KNO3 (high-NO3− leaves) nutrient solutions. PEPcase activity increased to 2.7-fold higher than that measured in dark-adapted tissue (control) during the first 60 minutes and continued to increase more slowly to 3.8-fold that of the control. This level was reached after 200 minutes exposure of the leaves to light and high NO3−. In contrast, the lower rate of increase recorded for low-NO3− leaves ceased after 60 minutes of exposure to light at 2.3-fold the control level. The short-term NO3− effect increased linearly with the level of NO3− uptake. In immunoprecipitation experiments, the antibody concentration for PEPcase precipitation increased with the protein extracts from the different treatments in the order: control, illuminated low-NO3− leaves, illuminated high-NO3− leaves. This order also applied with regard to a decreasing sensitivity to malate and an increasing stimulation by okadaic acid (an inhibitor of P-protein phosphatases). Following these studies, 32P labeling experiments were carried out in vivo. These showed that the light-induced change in the properties of the PEPcase was due to an alteration in the phosphorylation state of the protein and that this effect was enhanced in high-NO3− conditions. Based on the responses of PEPcase and sucrose phosphate synthase in wheat leaves to light and NO3−, an interpretation of the role of NO3− as either an inhibitor of P-protein phosphatase(s) or activator of protein kinase(s) is inferred. In the presence of NO3−, the phosphorylation state of both PEPcase and sucrose phosphate synthase is increased. This causes activation of the former enzyme and inhibition of the latter. We suggest that NO3− modulates the relative protein kinase/protein phosphatase ratio to favor increased phosphorylation of both enzymes in order to redirect carbon flow away from sucrose synthesis and toward amino acid synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Brulfert J., Vidal J., Le Marechal P., Gadal P., Queiroz O., Kluge M., Kruger I. Phosphorylation-dephosphorylation process as a probable mechanism for the diurnal regulatory changes of phosphoenolpyruvate carboxylase in CAM plants. Biochem Biophys Res Commun. 1986 Apr 14;136(1):151–159. doi: 10.1016/0006-291x(86)90889-2. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cretin C., Keryer E., Tagu D., Lepiniec L., Vidal J., Gadal P. Complete cDNA sequence of sorghum phosphoenolpyruvate carboxylase involved in C4 photosynthesis. Nucleic Acids Res. 1990 Feb 11;18(3):658–658. doi: 10.1093/nar/18.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. L., Huber S. C., Nielsen T. H. Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch Biochem Biophys. 1989 May 1;270(2):681–690. doi: 10.1016/0003-9861(89)90551-1. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T. Changes in Sensitivity to Effectors of Maize Leaf Phosphoenolypyruvate Carboxylase during Light/Dark Transitions. Plant Physiol. 1986 Jun;81(2):674–677. doi: 10.1104/pp.81.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J. A., Vidal J., Echevarría C., Chollet R. In vivo regulatory phosphorylation site in c(4)-leaf phosphoenolpyruvate carboxylase from maize and sorghum. Plant Physiol. 1991 May;96(1):297–301. doi: 10.1104/pp.96.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J., Echevarría C., Vidal J., Chollet R. Protein turnover as a component in the light/dark regulation of phosphoenolpyruvate carboxylase protein-serine kinase activity in C4 plants. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2712–2715. doi: 10.1073/pnas.88.7.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques I. A., Oberholzer M. J., Erismann K. H. Effects of Different Inorganic Nitrogen Sources on Photosynthetic Carbon Metabolism in Primary Leaves of Non-nodulated Phaseolus vulgaris L. Plant Physiol. 1983 Mar;71(3):555–561. doi: 10.1104/pp.71.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G., Hamilton I. D., Fewson C. A., Wilkins M. B. Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J. 1986 Oct 1;239(1):213–220. doi: 10.1042/bj2390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer P., Erismann K. H. Effect of Nitrate and Ammonium Nutrition of Nonnodulated Phaseolus vulgaris L. on Phosphoenolpyruvate Carboxylase and Pyruvate Kinase Activity. Plant Physiol. 1985 Jul;78(3):455–458. doi: 10.1104/pp.78.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Sugiharto B., Miyata K., Nakamoto H., Sasakawa H., Sugiyama T. Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaf. Plant Physiol. 1990 Apr;92(4):963–969. doi: 10.1104/pp.92.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe G. C., Schuller K. A., Smith R. G., Feil R., Plaxton W. C., Turpin D. H. Relationship between NH(4) Assimilation Rate and in Vivo Phosphoenolpyruvate Carboxylase Activity : Regulation of Anaplerotic Carbon Flow in the Green Alga Selenastrum minutum. Plant Physiol. 1990 Sep;94(1):284–290. doi: 10.1104/pp.94.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K., Meyer C. R. Activation of higher plant phosphoenolpyruvate carboxylases by glucose-6-phosphate. Plant Physiol. 1989 Jun;90(2):648–652. doi: 10.1104/pp.90.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]



